Abstract
Acute myeloid leukemia (AML) is a heterogeneous clonal disease characterized overall by an aggressive clinical course. The underlying genetic abnormalities present in leukemic cells contribute significantly to the AML phenotype. Mutations in FMS-like tyrosine kinase 3 (FLT3) are one of the most common genetic abnormalities identified in AML, and the presence of these mutations strongly influences disease presentation and negatively impacts prognosis. Since mutations in FLT3 were identified in AML, they have been recognized as a valid therapeutic target resulting in decades of research to develop effective small molecule inhibitor treatment that could improve outcome for these patients. Despite the approval of several FLT3 inhibitors over the last couple of years, the treatment of patients with FLT3-mutated AML remains challenging and many questions still need to be addressed. This review will provide an up-to-date overview of our current understanding of FLT3-mutated AML and discuss what the current status is of the available FLT3 inhibitors for the day-to-day management of this aggressive disease.

Similar content being viewed by others
Data availability
All figures are originals.
References
Collins FS, Varmus H (2015) A new initiative on precision medicine. N Engl J Med 372:793–795. https://doi.org/10.1056/NEJMp1500523
Fountzilas E, Tsimberidou AM, Vo HH, Kurzrock R (2022) Clinical trial design in the era of precision medicine. Genome Med 14:101. https://doi.org/10.1186/s13073-022-01102-1
Schwartzberg L, Kim ES, Liu D, Schrag D (2017) Precision oncology: who, how, what, when, and when not? Am Soc Clin Oncol Educ Book 37:160–169. https://doi.org/10.1200/EDBK_184176
Subbiah V, Kurzrock R (2018) Challenging standard-of-care paradigms in the precision oncology era. Trends in Cancer 4:101–109. https://doi.org/10.1016/j.trecan.2017.12.004
Thomas X (2019) Acute promyelocytic leukemia: a history over 60 years—from the most malignant to the most curable form of acute leukemia. Oncol Ther 7:33–65. https://doi.org/10.1007/s40487-018-0091-5
Kantarjian H, Kadia T, DiNardo C et al (2021) Acute myeloid leukemia: current progress and future directions. Blood Cancer J 11:41. https://doi.org/10.1038/s41408-021-00425-3
Burd A, Levine RL, Ruppert AS et al (2020) Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med 26:1852–1858. https://doi.org/10.1038/s41591-020-1089-8
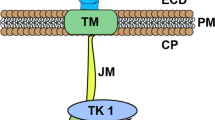
Gilliland DG, Griffin JD (2002) The roles of FLT3 in hematopoiesis and leukemia. Blood 100:1532–1542. https://doi.org/10.1182/blood-2002-02-0492
Kim ES (2017) Midostaurin: first flobal approval. Drugs 77:1251–1259. https://doi.org/10.1007/s40265-017-0779-0
Pulte ED, Norsworthy KJ, Wang Y et al (2021) FDA approval summary: gilteritinib for relapsed or tefractory acute myeloid leukemia with a FLT3 mutation. Clinical Cancer Research 27:3515–3521. https://doi.org/10.1158/1078-0432.CCR-20-4271
Food US, Administration D, (FDA). (2023) FDA approves quizartinib for newly diagnosed acute myeloid leukemia. News release Accessed September 10, 2023. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-quizartinib-newly-diagnosed-acute-myeloid-leukemia
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134. https://doi.org/10.1016/j.cell.2010.06.011
Rosnet O, Matteï M-G, Marchetto S, Birnbaum D (1991) Isolation and chromosomal localization of a novel FMS-like tyrosine kinase gene. Genomics 9:380–385. https://doi.org/10.1016/0888-7543(91)90270-O
Kikushige Y, Yoshimoto G, Miyamoto T et al (2008) Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/macrophage progenitor stages to maintain cell survival. J Immunol 180:7358–7367. https://doi.org/10.4049/jimmunol.180.11.7358
Mooney C, Cunningham A, Tsapogas P et al (2017) Selective expression of Flt3 within the mouse hematopoietic stem cell compartment. IJMS 18:1037. https://doi.org/10.3390/ijms18051037
Grafone T, Palmisano M, Nicci C, Storti S (2012) An overview on the role of FLT3-tyrosine kinase receptor in acute myeloid leukemia: biology and treatment. Oncol Rev 6:8. https://doi.org/10.4081/oncol.2012.e8
Griffith J, Black J, Faerman C et al (2004) The structural basis for auto-inhibition of FLT3 by the juxtamembrane domain. Mol Cell 13(2):169–178. https://doi.org/10.1016/s1097-2765(03)00505-7
Agnès F, Shamoon B, Dina C et al (1994) Genomic structure of the downstream part of the human FLT3 gene: exon/intron structure conservation among genes encoding receptor tyrosine kinases (RTK) of subclass III. Gene 145:283–288. https://doi.org/10.1016/0378-1119(94)90021-3
Takahashi S (2011) Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: biology and therapeutic implications. J Hematol Oncol 4:13. https://doi.org/10.1186/1756-8722-4-13
Birg F, Courcoul M, Rosnet O et al (1992) Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood 80:2584–2593. https://doi.org/10.1182/blood.V80.10.2584.2584
Nakao M, Yokota S, Iwai , et al (1996) Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 10(12): 1911-1918.
Yokota S, Kiyoi H, Nakao M et al (1997) Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia 11:1605–1609. https://doi.org/10.1038/sj.leu.2400812
Thiede C, Steudel C, Mohr B et al (2002) Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 99:4326–4335. https://doi.org/10.1182/blood.V99.12.4326
Kottaridis PD, Gale RE, Frew ME et al (2001) The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 98:1752–1759. https://doi.org/10.1182/blood.V98.6.1752
Kiyoi H, Naoe T, Yokota S et al (1997) Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia 11:1447–1452. https://doi.org/10.1038/sj.leu.2400756
Choudhary C, Brandts C, Schwable J et al (2007) Activation mechanisms of STAT5 by oncogenic Flt3-ITD. Blood 110:370–374. https://doi.org/10.1182/blood-2006-05-024018
Mizuki M, Fenski R, Halfter H et al (2000) Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 96:3907–3914. https://doi.org/10.1182/blood.V96.12.3907
Mead AJ, Linch DC, Hills RK et al (2007) FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood 110:1262–1270. https://doi.org/10.1182/blood-2006-04-015826
Yanada M, Matsuo K, Suzuki T et al (2005) Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia 19:1345–1349. https://doi.org/10.1038/sj.leu.2403838
Gale RE, Green C, Allen C et al (2008) The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 111:2776–2784. https://doi.org/10.1182/blood-2007-08-109090
Schlenk RF, Kayser S, Bullinger L et al (2014) Differential impact of allelic ratio and insertion site in FLT3-ITD–positive AML with respect to allogeneic transplantation. Blood 124:3441–3449. https://doi.org/10.1182/blood-2014-05-578070
Dohner H, Wei AH, Appelbaum FR, et al (2022) Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 140(12): 1345-1377. https:http://doi.org/10.1182/blood.2022016867.
Grundler R, Miething C, Thiede C et al (2005) FLT3-ITD and tyrosine kinase domain mutants induce 2 distinct phenotypes in a murine bone marrow transplantation model. Blood 105:4792–4799. https://doi.org/10.1182/blood-2004-11-4430
Mupo A, Celani L, Dovey O et al (2013) A powerful molecular synergy between mutant Nucleophosmin and Flt3-ITD drives acute myeloid leukemia in mice. Leukemia 27:1917–1920. https://doi.org/10.1038/leu.2013.77
Shih AH, Jiang Y, Meydan C et al (2015) Mutational cooperativity linked to combinatorial epigenetic gain of function in acute myeloid leukemia. Cancer Cell 27:502–515. https://doi.org/10.1016/j.ccell.2015.03.009
Smith CC, Wang Q, Chin C-S et al (2012) Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 485:260–263. https://doi.org/10.1038/nature11016
Larrosa-Garcia M, Baer MR (2017) FLT3 Inhibitors in acute myeloid leukemia: current status and future directions. Molecular Cancer Therapeutics 16:991–1001. https://doi.org/10.1158/1535-7163.MCT-16-0876
Ke Y-Y, Singh VK, Coumar MS et al (2015) Homology modeling of DFG-in FMS-like tyrosine kinase 3 (FLT3) and structure-based virtual screening for inhibitor identification. Sci Rep 5:11702. https://doi.org/10.1038/srep11702
Fiedler W, Mesters R, Tinnefeld H et al (2003) A phase 2 clinical study of SU5416 in patients with refractory acute myeloid leukemia. Blood 102:2763–2767. https://doi.org/10.1182/blood-2002-10-2998
Giles FJ, Stopeck AT, Silverman LR et al (2003) SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood 102:795–801. https://doi.org/10.1182/blood-2002-10-3023
Fiedler W, Serve H, Döhner H et al (2005) A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood 105:986–993. https://doi.org/10.1182/blood-2004-05-1846
Fiedler W, Kayser S, Kebenko M et al (2015) A phase I/II study of sunitinib and intensive chemotherapy in patients over 60 years of age with acute myeloid leukaemia and activating FLT3 mutations. Br J Haematol 169:694–700. https://doi.org/10.1111/bjh.13353
Knapper S, Mills KI, Gilkes AF et al (2006) The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: the induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood 108:3494–3503. https://doi.org/10.1182/blood-2006-04-015487
Levis M, Ravandi F, Wang ES et al (2011) Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood 117:3294–3301. https://doi.org/10.1182/blood-2010-08-301796
Knapper S, Russell N, Gilkes A et al (2017) A randomized assessment of adding the kinase inhibitor lestaurtinib to first-line chemotherapy for FLT3-mutated AML. Blood 129:1143–1154. https://doi.org/10.1182/blood-2016-07-730648
Borthakur G, Kantarjian H, Ravandi F et al (2011) Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica 96:62–68. https://doi.org/10.3324/haematol.2010.030452
Ravandi F, Alattar ML, Grunwald MR et al (2013) Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 121:4655–4662. https://doi.org/10.1182/blood-2013-01-480228
Uy GL, Mandrekar SJ, Laumann K et al (2017) A phase 2 study incorporating sorafenib into the chemotherapy for older adults with FLT3-mutated acute myeloid leukemia: CALGB 11001. Blood Advances 1:331–340. https://doi.org/10.1182/bloodadvances.2016003053
Röllig C, Serve H, Hüttmann A et al (2015) Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. The Lancet Oncology 16:1691–1699. https://doi.org/10.1016/S1470-2045(15)00362-9
Loo S, Roberts AW, Anstee NS et al (2023) Sorafenib plus intensive chemotherapy in newly diagnosed FLT3-ITD AML: a randomized, placebo-controlled study by ALLG. Blood Aug 30:blood.2023020301. Online ahead of print. https://doi.org/10.1182/blood.2023020301
Stone RM, Fischer T, Paquette R et al (2012) Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia 26:2061–2068. https://doi.org/10.1038/leu.2012.115
Fischer T, Stone RM, DeAngelo DJ, et al (2010) Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. JCO 28:4339–4345. https://doi.org/10.1200/JCO.2010.28.9678
Stone RM, Mandrekar SJ, Sanford BL et al (2017) Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377:454–464. https://doi.org/10.1056/NEJMoa1614359
Daver N, Schlenk RF, Russell NH, Levis MJ (2019) Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33:299–312. https://doi.org/10.1038/s41375-018-0357-9
Weisberg E, Roesel J, Furet P et al (2010) Antileukemic effects of novel first- and second-generation FLT3 inhibitors: structure-affinity comparison. Genes & Cancer 1:1021–1032. https://doi.org/10.1177/1947601910396505
Cortes JE, Kantarjian H, Foran JM, et al (2013) Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3–internal tandem duplication status. JCO 31:3681–3687. https://doi.org/10.1200/JCO.2013.48.8783
Cortes J, Perl AE, Döhner H et al (2018) Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 19:889–903. https://doi.org/10.1016/S1470-2045(18)30240-7
Cortes JE, Tallman MS, Schiller GJ et al (2018) Phase 2b study of 2 dosing regimens of quizartinib monotherapy in FLT3-ITD–mutated, relapsed or refractory AML. Blood 132:598–607. https://doi.org/10.1182/blood-2018-01-821629
Cortes JE, Khaled S, Martinelli G et al (2019) Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 20:984–997. https://doi.org/10.1016/S1470-2045(19)30150-0
Erba HP, Montesinos P, Kim HJ et al (2023) Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukemia (QuANTUM-FIRST): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401(10388):1571–1583. https://doi.org/10.1016/s0140-6737(23)00464-6
Usuki K, Sakura T, Kobayashi Y et al (2018) Clinical profile of gilteritinib in Japanese patients with relapsed/refractory acute myeloid leukemia: an open-label phase 1 study. Cancer Sci 109:3235–3244. https://doi.org/10.1111/cas.13749
Perl AE, Altman JK, Cortes J et al (2017) Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol 18:1061–1075. https://doi.org/10.1016/S1470-2045(17)30416-3
Perl AE, Martinelli G, Cortes JE et al (2019) Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 381:1728–1740. https://doi.org/10.1056/NEJMoa1902688
Smolich BD, Yuen HA, West KA et al (2001) The antiangiogenic protein kinase inhibitors SU5416 and SU6668 inhibit the SCF receptor (c-kit) in a human myeloid leukemia cell line and in acute myeloid leukemia blasts. Blood 97:1413–1421. https://doi.org/10.1182/blood.V97.5.1413
Levis M, Pham R, Smith BD, Small D (2004) In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood 104:1145–1150. https://doi.org/10.1182/blood-2004-01-0388
Voso MT, Larson RA, Jones D et al (2020) Midostaurin in patients with acute myeloid leukemia and FLT3-TKD mutations: a subanalysis from the RATIFY trial. Blood Advances 4:4945–4954. https://doi.org/10.1182/bloodadvances.2020002904
Larson RA, Mandrekar SJ, Huebner LJ et al (2021) Midostaurin reduces relapse in FLT3-mutant acute myeloid leukemia: the Alliance CALGB 10603/RATIFY trial. Leukemia 35:2539–2551. https://doi.org/10.1038/s41375-021-01179-4
Kampa-Schittenhelm KM, Heinrich MC, Akmut F et al (2013) Quizartinib (AC220) is a potent second generation class III tyrosine kinase inhibitor that displays a distinct inhibition profile against mutant-FLT3, -PDGFRA and -KIT isoforms. Mol Cancer 12:19. https://doi.org/10.1186/1476-4598-12-19
Lee LY, Hernandez D, Rajkhowa T et al (2017) Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood 129:257–260. https://doi.org/10.1182/blood-2016-10-745133
Mori M, Kaneko N, Ueno Y et al (2017) Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia. Invest New Drugs 35:556–565. https://doi.org/10.1007/s10637-017-0470-z
Smith CC, Lasater EA, Lin KC et al (2014) Crenolanib is a selective type I pan-FLT3 inhibitor. Proc Natl Acad Sci USA 111:5319–5324. https://doi.org/10.1073/pnas.1320661111
Zimmerman EI, Turner DC, Buaboonnam J et al (2013) Crenolanib is active against models of drug-resistant FLT3-ITD−positive acute myeloid leukemia. Blood 122:3607–3615. https://doi.org/10.1182/blood-2013-07-513044
Bazarbachi A, Bug G, Baron F et al (2020) Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3 -internal tandem duplication: a position statement from the acute leukemia working party of the European Society for Blood and Marrow Transplantation. Haematologica 105:1507–1516. https://doi.org/10.3324/haematol.2019.243410
Xuan L, Wang Y, Huang F et al (2018) Effect of sorafenib on the outcomes of patients with FLT3-ITD acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation: Effect of Sorafenib on FLT3-ITD AML With HSCT. Cancer 124:1954–1963. https://doi.org/10.1002/cncr.31295
Battipaglia G, Massoud R, Ahmed SO et al (2019) Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 mutated acute myeloid leukemia: an update. Clin Lymphoma Myeloma Leuk 19:506–508. https://doi.org/10.1016/j.clml.2019.04.004
Battipaglia G, Ruggeri A, Massoud R et al (2017) Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms-like tyrosine kinase 3-mutated acute myeloid leukemia: sorafenib after HSCT for FLT3-mutated AML. Cancer 123:2867–2874. https://doi.org/10.1002/cncr.30680
Burchert A, Bug G, Fritz LV, et al (2020) Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3 –internal tandem duplication mutation (SORMAIN). JCO 38:2993–3002. https://doi.org/10.1200/JCO.19.03345
Xuan L, Wang Y, Yang K et al (2023) Sorafenib mainteance after allogeneic haemopoietic stem-cell transplantation in patients with FLT3-ITD acute myeloid leukaemia: long-term follow-up of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol 10(8):e600–e611. https://doi.org/10.1016/S2352-3026(23)001117-5
Man CH, Fung TK, Ho C et al (2012) Sorafenib treatment of FLT3-ITD+ acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood 119:5133–5143. https://doi.org/10.1182/blood-2011-06-363960
Weinstein IB (2002) Addiction to oncogenes--the Achilles heal of cancer. Science 297:63–64. https://doi.org/10.1126/science.1073096
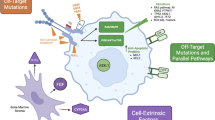
Weisberg E, Barrett R, Liu Q et al (2015) FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Update 12(3):81–89. https://doi.org/10.1016/j.drup.2009.04.001
Scholl S, Fleischmann M, Schnetzke U et al (2020) Molecular mechanisms of resistance to FLT3 inhibitors in acute myeloid leukemia: ongoing challenges and future treatments. Cells 9(11):2493. https://doi.org/10.3390/cells9112493
Heidel F, Solem FK, Breitenbucher F et al (2006) Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood 107(1):293–300. https://doi.org/10.1182/blood-2005006-2469
Breitenbuecher F, Markova B, Kaspar S et al (2009) A novel molecular mechanism of primary resistance to FLT3 kinase inhibitors in AML. Blood 113(17):4063–4073. https://doi.org/10.1182/blood-2007-11-12664
Baker SD, Zimmerman EI, Wang Y-D et al (2013) Emergence of polyclonal FLT3 tyrosine kinase domain mutations during sequential therapy with sorafenib and sunitinib in FLT3-ITD–positive acute myeloid leukemia. Clin Cancer Res 19:5758–5768. https://doi.org/10.1158/1078-0432.CCR-13-1323
Albers C, Leischner H, Verbeek M et al (2013) The secondary FLT3-ITD F691L mutation induces resistance to AC220 in FLT3-ITD+ AML but retains in vitro sensitivity to PKC412 and Sunitinib. Leukemia 27:1416–1418. https://doi.org/10.1038/leu.2013.14
Smith CC, Paguirigan A, Jeschke GR et al (2017) Heterogeneous resistance to quizartinib in acute myeloid leukemia revealed by single-cell analysis. Blood 130:48–58. https://doi.org/10.1182/blood-2016-04-711820
Smith CC, Zhang C, Lin KC et al (2015) Characterizing and overriding the structural mechanism of the quizartinib-resistant FLT3 “gatekeeper” F691L mutation with PLX3397. Cancer Discov 5:668–679. https://doi.org/10.1158/2159-8290.CD-15-0060
Kiyoi H, Kawashima N, Ishikawa Y (2020) FLT3 mutations in acute myeloid leukemia: therapeutic paradigm beyond inhibitor development. Cancer Sci 11192:312–322. https://doi.org/10.1111/cas.14274
Zhang Y, Wang P, Wang Y (2023) Sitravatinib as a potent FLT3 inhibitor can overcome gilteritinib resistance in acute myeloid leukemia. Biomarker Res 11(1):8. https://doi.org/10.1186/s40364-022-00447-4
McMahon CM, Ferng T, Canaani J et al (2019) Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov 9:1050–1063. https://doi.org/10.1158/2159-8290.CD-18-1453
Ghiaur G, Levis M (2017) Mechanisms of resistance to FLT3 inhibitors and the role of the bone marrow microenvironment. Hematol Oncol Clin North Am 31:681–692. https://doi.org/10.1016/j.hoc.2017.04.005
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. https://doi.org/10.1126/science.284.5411.143
Yang X, Sexauer A, Levis M (2014) Bone marrow stroma-mediated resistance to FLT3 inhibitors in FLT3-ITD AML is mediated by persistent activation of extracellular regulated kinase. Br J Haematol 164:61–72. https://doi.org/10.1111/bjh.12599
Traer E, Martinez J, Javidi-Sharifi N et al (2016) FGF2 from marrow microenvironment promotes resistance to FLT3 inhibitors in acute myeloid leukemia. Cancer Res 76:6471–6482. https://doi.org/10.1158/0008-5472.CAN-15-3569
Sato T, Yang X, Knapper S et al (2011) FLT3 ligand impeded the efficacy of FLT3 inhibitors in vitro and in vivo. Blood 1117(12):3288–3293. https://doi.org/10.1182/blood-2010-01-2667242
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lap, C.J., Abrahim, M.S. & Nassereddine, S. Perspectives and challenges of small molecule inhibitor therapy for FLT3-mutated acute myeloid leukemia. Ann Hematol (2023). https://doi.org/10.1007/s00277-023-05545-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-023-05545-3