Abstract
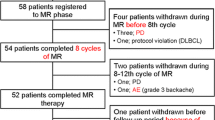
The anti-PD-1 antibodies have been reported to show a striking effect in relapsed and refractory(R/R) classical Hodgkin lymphoma (cHL), however, there is still limited real-world data assessing the role of anti-PD-1 antibody monotherapy in early-stage cHL. In this retrospective analysis, we reported the effectiveness and safety of tislelizumab monotherapy in the first-line therapy of early-stage cHL. Twenty-three consecutive patients (10 males and 13 females) with previously untreated stage I A-II B cHL were included. At interim evaluation after 2 doses of tislelizumab monotherapy, 11 of 23 patients (47.8%) achieved complete response (CR). At the end of tislelizumab monotherapy (EOTM), objective response was observed in 22 of 23 patients (95.7%), with CR in 16 patients (69.6%). Among six patients with PR-EOTM, two patients underwent 4 cycles of ABVD chemotherapy and one patient underwent 4 cycles of tislelizumab plus AVD. One patient who developed progressive disease (PD) after 4 doses of tislelizumab subsequently underwent 4 cycles of ABVD chemotherapy. Except for four patients with CR-EOTM, consolidative radiotherapy was given to 19 patients. All patients obtained CR at the end of all treatments. With a median follow-up time of 21.3 months (range, 6.9–32.7 months), the estimated 2-year PFS rate and 2-year OS rate were 95.65% and 100%, respectively. Except for grade 3 lymphocyte count decreased, no other grade 3/4 TRAE was observed. In addition, no serious AE was reported. Our preliminary data observed that tislelizumab monotherapy was safe and highly effective in previously untreated early-stage cHL.



Similar content being viewed by others
References
Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, Armand P, Chapuy B, de Jong D, Hoppe RT, Neuberg DS, Rodig SJ, Shipp MA (2016) PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 34:2690–2697. https://doi.org/10.1200/JCO.2016.66.4482
Carey CD, Gusenleitner D, Lipschitz M, Roemer M, Stack EC, Gjini E, Hu X, Redd R, Freeman GJ, Neuberg D, Hodi FS, Liu XS, Shipp MA, Rodig SJ (2017) Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 130:2420–2430. https://doi.org/10.1182/blood-2017-03-770719
Shanbhag S, Ambinder RF (2018) Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin 68:116–132. https://doi.org/10.3322/caac.21438
Myrehaug S, Pintilie M, Tsang R, Mackenzie R, Crump M, Chen Z, Sun A, Hodgson DC (2008) Cardiac morbidity following modern treatment for Hodgkin lymphoma: supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma 49:1486–1493. https://doi.org/10.1080/10428190802140873
Lorigan P, Radford J, Howell A, Thatcher N (2005) Lung cancer after treatment for Hodgkin’s lymphoma: a systematic review. Lancet Oncol 6:773–779. https://doi.org/10.1016/S1470-2045(05)70387-9
Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, Tomita A, von Tresckow B, Shipp MA, Lin J, Kim E, Nahar A, Balakumaran A, Moskowitz CH (2019) Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 134:1144–1153. https://doi.org/10.1182/blood.2019000324
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, De Boer JP, Kuruvilla J, Savage KJ, Trneny M, Shipp MA, Kato K, Sumbul A, Farsaci B, Ansell SM (2018) Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol 36:1428–1439. https://doi.org/10.1200/JCO.2017.76.0793
Ramchandren R, Domingo-Domènech E, Rueda A, Trněný M, Feldman TA, Lee HJ, Provencio M, Sillaber C, Cohen JB, Savage KJ, Willenbacher W, Ligon AH, Ouyang J, Redd R, Rodig SJ, Shipp MA, Sacchi M, Sumbul A, Armand P, Ansell SM (2019) Nivolumab for newly diagnosed advanced-stage classic Hodgkin lymphoma: safety and efficacy in the phase II CheckMate 205 Study. J Clin Oncol 37:1997–2007. https://doi.org/10.1200/JCO.19.00315
Bröckelmann PJ, Bühnen I, Meissner J, Trautmann-Grill K, Herhaus P, Halbsguth TV, Schaub V, Kerkhoff A, Mathas S, Bormann M, Dickhut A, Kaul H, Fuchs M, Kobe C, Baues C, Borchmann P, Engert A, von Tresckow B (2023) Nivolumab and doxorubicin, vinblastine, and dacarbazine in early-stage unfavorable Hodgkin lymphoma: final analysis of the randomized German Hodgkin study group phase II NIVAHL trial. J Clin Oncol 41:1193–1199. https://doi.org/10.1200/JCO.22.02355
Allen PB, Savas H, Evens AM, Advani RH, Palmer B, Pro B, Karmali R, Mou E, Bearden J, Dillehay G, Bayer RA, Eisner RM, Chmiel JS, O’Shea K, Gordon LI, Winter JN (2021) Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma. Blood 137:1318–1326. https://doi.org/10.1182/blood.2020007400
Chen J, Zhang H, Zhu L, Zhao Y, Ding Y, Yuan Y (2020) Tislelizumab for the treatment of classical Hodgkin’s lymphoma. Drugs Today (Barc) 56:781–785. https://doi.org/10.1358/dot.2020.56.12.3233362
Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D, Li W, Yang H, Liu T, Wang Q, Lv F, Guo H, Zhao X, Wang D, Zhang P, Wang Y, Wang L, Liu T, Zhang Y, Shen Z, Huang J, Zhu J (2022) Tislelizumab for relapsed/refractory classical Hodgkin lymphoma: 3-year follow-up and correlative biomarker analysis. Clin Cancer Res 28:1147–1156. https://doi.org/10.1158/1078-0432.CCR-21-2023
Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, Zhang Y, Zhou X, Wang Z, Wang Y, Shi Y, Bai H, Liu N, Yang X, Cui X, Cao Y, Liu Q, Song J, Li Y, Tang Z, Guo M, Wang L, Li K (2018) The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 67:1079–1090. https://doi.org/10.1007/s00262-018-2160-x
Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV (2015) FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell 28:285–295. https://doi.org/10.1016/j.ccell.2015.08.004
Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D, Li W, Yang H, Liu T, Wang Q, Lv F, Guo H, Yang L, Elstrom R, Huang J, Novotny W, Wei V, Zhu J (2020) Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia 34:533–542. https://doi.org/10.1038/s41375-019-0545-2
Ansell SM, Bröckelmann PJ, von Keudell G, Lee HJ, Santoro A, Zinzani PL, Collins GP, Cohen JB, de Boer JP, Kuruvilla J, Savage KJ, Trněný M, Provencio M, Jaeger U, Willenbacher W, Wen R, Akyol A, Mikita-Geoffroy J, Shipp MA, Engert AM, Armand P (2023) Nivolumab for relapsed/refractory classical Hodgkin lymphoma: 5-year survival from pivotal phase 2 CheckMate 205 study. Blood Adv. https://doi.org/10.1182/bloodadvances.2023010334
Ding K, Liu H, Ma J, Yang H, Cao L, Wang H, Peng H, Shi W, Zhao X, Wu W, Zhu H, Li J, Fan L (2023) Tislelizumab with gemcitabine and oxaliplatin in patients with relapsed or refractory classic Hodgkin lymphoma: a multicenter phase II trial. Haematologica 108:2146–2154. https://doi.org/10.3324/haematol.2022.282266
Bröckelmann PJ, Goergen H, Keller U, Meissner J, Ordemann R, Halbsguth TV, Sasse S, Sökler M, Kerkhoff A, Mathas S, Hüttmann A, Bormann M, Zimmermann A, Mettler J, Fuchs M, von Tresckow B, Baues C, Rosenwald A, Klapper W, Kobe C, Borchmann P, Engert A (2020) Efficacy of nivolumab and AVD in early-stage unfavorable classic Hodgkin lymphoma: the randomized phase 2 German Hodgkin study group NIVAHL trial. JAMA Oncol 6:872–880. https://doi.org/10.1001/jamaoncol.2020.0750
Chohan KL, Young JR, Lester S, Alhaj Moustafa M, Rosenthal A, Tun HW, Hoppe BS, Johnston PB, Micallef IN, Habermann TM, Ansell SM (2022) A real-world study of combined modality therapy for early-stage Hodgkin lymphoma: too little treatment impacts outcome. Blood Adv 6:4241–4250. https://doi.org/10.1182/bloodadvances.2022007363
Myint ZW, Shrestha R, Siddiqui S, Slone S, Huang B, Ramlal R, Monohan GP, Hildebrandt GC, Saeed H (2020) Ten-year survival outcomes for patients with early stage classical Hodgkin lymphoma: an analysis from Kentucky Cancer Registry. Hematol Oncol Stem Cell Ther 13:17–22. https://doi.org/10.1016/j.hemonc.2019.08.009
Hanel W, Herrera AF, Epperla N (2022) Management of classical Hodgkin lymphoma: a look at up to date evidence and current treatment approaches. Exp Hematol Oncol 11:108. https://doi.org/10.1186/s40164-022-00360-4
Fuchs M, Goergen H, Kobe C, Kuhnert G, Lohri A, Greil R, Sasse S, Topp MS, Schäfer E, Hertenstein B, Soekler M, Vogelhuber M, Zijlstra JM, Keller UB, Krause SW, Wilhelm M, Maschmeyer G, Thiemer J, Dührsen U, Meissner J, Viardot A, Eich H, Baues C, Diehl V, Rosenwald A, von Tresckow B, Dietlein M, Borchmann P, Engert A (2019) Positron emission tomography-guided treatment in early-stage favorable Hodgkin lymphoma: final results of the international, randomized phase III HD16 trial by the German Hodgkin study group. J Clin Oncol 37:2835–2845. https://doi.org/10.1200/JCO.19.00964
Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, Wimperis J, Culligan D, Popova B, Smith P, McMillan A, Brownell A, Kruger A, Lister A, Hoskin P, O’Doherty M, Barrington S (2015) Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med 372:1598–1607. https://doi.org/10.1056/NEJMoa1408648
Kumar A, Casulo C, Yahalom J, Schöder H, Barr PM, Caron P, Chiu A, Constine LS, Drullinsky P, Friedberg JW, Gerecitano JF, Hamilton A, Hamlin PA, Horwitz SM, Jacob AG, Matasar MJ, McArthur GN, McCall SJ, Moskowitz AJ, Noy A, Palomba ML, Portlock CS, Straus DJ, VanderEls N, Verwys SL, Yang J, Younes A, Zelenetz AD, Zhang Z, Moskowitz CH (2016) Brentuximab vedotin and AVD followed by involved-site radiotherapy in early stage, unfavorable risk Hodgkin lymphoma. Blood 128:1458–1464. https://doi.org/10.1182/blood-2016-03-703470
Li Y, Sun H, Yan Y, Sun T, Wang S, Ma H (2018) Long-term survival rates of patients with stage III-IV Hodgkin lymphoma according to age, sex, race, and socioeconomic status, 1984–2013. Oncologist 23:1328–1336. https://doi.org/10.1634/theoncologist.2017-0541
Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, Gelber RD, Goldhirsch A (2018) Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 19:737–746. https://doi.org/10.1016/S1470-2045(18)30261-4
Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ (2015) Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373–377. https://doi.org/10.1038/nature14292
Chen D, Verma V, Patel RR, Barsoumian HB, Cortez MA, Welsh JW (2020) Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int J Radiat Oncol Biol Phys 108:196–203. https://doi.org/10.1016/j.ijrobp.2020.01.032
Zhao Q, Bi Y, Xue J, Liu Y, Zhu J, Qin S (2022) Prognostic value of absolute lymphocyte count in patients with advanced esophageal cancer treated with immunotherapy: a retrospective analysis. Ann Transl Med 10:744. https://doi.org/10.21037/atm-22-2669
Huemer F, Lang D, Westphal T, Gampenrieder SP, Hutarew G, Weiss L, Hackl H, Lamprecht B, Rinnerthaler G, Greil R (2019) Baseline absolute lymphocyte count and ECOG performance score are associated with survival in advanced non-small cell lung cancer undergoing PD-1/PD-L1 blockade. J Clin Med 8:1014. https://doi.org/10.3390/jcm8071014
Lee WJ, Wang YL, Peng HH, Lin CT (2023) Increased absolute lymphocyte count, increased absolute neutrophil count and low platelet to lymphocyte ratio as predicting factors in the superior disease control of refractory/relapsing gynecologic malignancies with anti PD-1 therapy: 10 years of experience in a single institution. Taiwan J Obstet Gynecol 62:506–509. https://doi.org/10.1016/j.tjog.2022.08.021
Van Heertum RL, Scarimbolo R, Wolodzko JG, Klencke B, Messmann R, Tunc F, Sokol L, Agarwal R, Strafaci JA, O’Neal M (2017) Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: an operational approach for clinical trials. Drug Des Devel Ther 11:1719–1728. https://doi.org/10.2147/DDDT.S136988
Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, Hoos A, Barrington SF, Armand P (2016) Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 128:2489–2496. https://doi.org/10.1182/blood-2016-05-718528
Acknowledgements
We thank all the patients included in this study and their families, caregivers, and friends.
Funding
This work was supported by grants from National Science and Technology Major Project (nos. 2018ZX09734003), the National Natural Science Foundation of China (nos.81872902, 82073917, 82103579, 82104273).
Author information
Authors and Affiliations
Contributions
ZML had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. PS, HY, BTZ and YW were involved in the study concepts and design. PS, BTZ, KMH and MN performed the statistical analysis and drafted the manuscript. All authors read, critically revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest disclosure
All authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, P., Yang, H., Wang, Y. et al. Tislelizumab monotherapy in patients with previously untreated early-stage classical Hodgkin lymphoma: a real-world study. Ann Hematol 103, 793–801 (2024). https://doi.org/10.1007/s00277-023-05541-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05541-7