Abstract
We performed a molecular analysis of formalin-fixed paraffin embedded and decalcified bone marrow trephine biopsies of 41 patients with a B-cell disorder with lymphoplasmacytic differentiation to enable a more precise diagnosis and to describe potentially prognostic and therapeutic relevant mutations. Analysis was performed with a commercially available next-generation sequencing (NGS) lymphoma panel (Lymphoma Solution, SophiaGenetics). Results were correlated with clinical and pathological parameters. Our group covered a spectrum of B-cell disorders with plasmacytic differentiation ranging from Waldenstroem’s macroglobulinemia (WM), to small-B-cell lymphomas with plasmacytic differentiation (SBCL-PC) to IgM myeloma (MM). The most helpful diagnostic criteria included morphology and immuno-phenotype as a prerequisite for the interpretation of molecular analysis. MYD88 mutation was present in nearly all WM, but also in 50% of SBCL-PCs, while MM were consistently negative. Driver mutations, such as TP53, were already detectable early in the course of the respective diseases indicating a higher risk of progression, transformation, and reduced progression-free survival. In addition, we report on a novel BIRC3 frameshift mutation in one case of a progressive WM. Our data indicate that patients with LPL/WM might benefit from thorough pathological work-up and detailed molecular analysis in terms of precise diagnosis and targeted treatment allocation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymphoplasmacytic lymphoma (LPL) is a B-cell neoplasia defined by a variable mixture of lymphocytes, lymphocytes with plasmacytic differentiation and plasma cells. It is known as Waldenstroem’s macroglobulinemia (WM), when presenting with IgM paraproteinemia and bone marrow involvement [1, 2]. However, (lympho)plasmacytic differentiation can also be found in other low-grade B-cell lymphomas, mainly in marginal zone lymphoma (MZL) or chronic lymphatic leukemia (CLL), but also in mantle cell lymphoma (MCL) [1, 3,4,5,6]. Beside morphological characteristics, the immune phenotype may overlap between low-grade lymphomas, resulting in clear diagnostic complexities [3, 4]. In fact, LPL/WM has no distinctive immune phenotype, differentiating it from MZL making a clear diagnosis nearly impossible [3]. This in turn leads to a diagnosis of a small B-cell lymphoma with plasmacytic differentiation (SBCL-PC) [4]. In addition, elevated IgM levels, lymphoid differentiation, and overlapping immune phenotype can also be seen in MM [7,8,9]. The detection of MYD88 mutations, most frequently the MYD88 L265P mutation, which is found in more than 90% of WM, was believed to reduce this diagnostic dilemma [4, 10]. However, while LPL/WM can be negative for MYD88 mutations, rare cases of MZL, CLL, and even MCL can harbor a MYD88 mutations, while only MMs are consistently negative [1, 3, 4]. A variety of clinical studies has dealt with the impact of molecular genetics in LPL/WM [5, 11,12,13,14,15,16]. The vast majority of WM demonstrates MYD88 mutations associated in up to 40% of cases with mutations on CXCR4 [5]. Lack of MYD88 mutation in LPL/WM, however, predicts worse outcome with higher risk of transformation, therapy-related myelodysplastic syndrome, as well as shorter time to transformation compared to MYD88 mutated patients [5, 11, 12]. Responses in MYD88-wild-type patients seem to be even worse with respect to BTK-inhibitors with the possible exception of zanubrutinib [5, 11, 12, 17]. While MYD88 is nearly always mutated in L265P, up to 40 different CXCR4 mutations to date occur in LPL/WM [5]. CXCR4 mutated cases have high serum IgM levels resulting more frequently in hyperviscosity of blood [5, 14]. In addition, the depth of response and progression-free survival partly depends on CXCR4 mutations when receiving BTK inhibitor-based treatment or Venetoclax, the first representative BCL-2 inhibitor [5, 13,14,15, 18]. No such impact is observed using upfront treatment with proteasome inhibitors [16]. Similarly, TP53 mutations, though only rarely found in WM are associated with poor outcome, while its role in resistance to BTK-inhibitor therapy is still under investigation [14, 19,20,21,22]. Further alterations found in LPL/WM include mutations on KMT2D, ARID1A, CD79A and B, NOTCH2, PRDM1, and TRAF3, but their impact on prognosis and treatment remains to be determined [14, 23, 24].
We have previously dealt with the presence of MYD88 L265P mutation in LPL/WM [10, 25]. We showed that digital PCR is a sensitive tool to detect this mutation in formalin-fixed paraffin-embedded (FFPE) and decalcified bone marrow trephine biopsies of patients with LPL/WM [25]. In the present study, we attempted to integrate morphology and molecular pathology for a more precise diagnosis by performing next-generation sequencing (NGS) in primary and relapsed LPL/WM. Our results indicate that patients might benefit from a broader molecular analysis already upon diagnosis to allocate the appropriate treatment.
Material and methods
Patients
Forty-one patients were identified either from the database of the University Clinics of Internal Medicine V, Hematology and Oncology or from the Institute of Pathology, Neuropathology and Molecular Pathology. The patients have been diagnosed with a neoplastic B-cell disorder presenting with a plasmacytic or lymphoplasmacytic differentiation between 1993 and 2016. Bone marrow trephine biopsies were collected and re-evaluated, including follow-up biopsies, if available. Clinical data such as IgM level at diagnosis, presence or absence of osteolysis, cytogenetics and information about disease progression or transformation were assessed from the patient chart.
The study was conducted according to the ICH-GCP guidelines and the declaration of Helsinki. Ethical approval was obtained from the ethical committee of the Medical University of Innsbruck (EK-Nr.1362/2020).
Re-evaluation and immunohistochemistry
A careful review was performed of all bone marrow biopsies including routinely performed immunohistochemistry to assess morphological parameters such as infiltration pattern, volume of infiltration and number of mast cells and immune-phenotype.
Pattern of infiltration was defined according to Garcia-Reyero J et al. [26] as:
-
Paratrabecular and interstitial, either patchy or nodular and/or diffuse
-
Only interstitial, either patchy or nodular or diffuse or diffuse-solid
A mast cell count on routinely performed Giemsa stains was done in five representative high-power fields (HPFs) in each biopsy.
Re-evaluation of immunohistochemistry included presence or absence of aberrant marker expression such as CD23 or CD5, positivity for plasma cell markers and evaluation of clonality using either immunohistochemistry or chromogen in situ hybridization (Ventana, Tucson, USA) on an automated immunostainer (Benchmarks Ultra, Ventana, Tucson, USA).
DNA isolation
DNA from FFPE tissue was extracted using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). DNA isolation was conducted according to the manufacturer’s instructions. Briefly, 3 × 5 µm slices or 6 × 5 µm slices (depending on the size of the tissue) were prepared in DNAse- and RNAse-free Eppendorf tubes. Paraffin was removed by a Xylol wash. The samples were lysed by use of proteinase K and heat incubated at 90 °C to reposition formalin crosslinking. Then the samples were transferred into QIAamp MinElute columns possessing special DNA binding properties. Residual impurities were washed away by use of the provided buffers. Finally, the respective DNA was eluted from the column membrane. The pure and concentrated DNA samples were stored at − 20 °C until further use.
Next-generation sequencing
NGS was performed using a commercially available lymphoma panel (Lymphoma Solution, SophiaGenetics, Geneva, Switzerland). This panel includes 54 genes relevant for lymphomagenesis, including those found in lymphomas with plasmacytic differentiation, such as MYD88, CXCR4, ARID1A, KMT2D, and those associated with disease progression or transformation such as TP53. Data were evaluated using the platform provided by SOPHIA GENETICS (SOPHIA DDM version 5.10.28.2). A cutoff of 5% was determined for the variant fraction in order to be regarded as a reliable mutation.
Statistics
For statistical analysis, SPSS 26 software was used. To test for correlations with clinical-pathological parameters the chi-square test was used. To determine the optimal cutoffs for continuous variables (such as number of mast cells) considering diagnostic ability, progression, transformation, and overall survival ROC analysis was performed and AUC was interpreted as follows: 0.5–0.6 = no discrimination, 0.6–0.7 = poor, 0.7 to 0.8 = acceptable, 0.8 to 0.9 = excellent, > 0.9 = outstanding. The optimal cutoffs were calculated using Youden´s index if AUC was > 0.7 [27, 28] (see suppl. Figure 1 and suppl. Table 1). Survival analysis was performed using Kaplan–Meier survival analysis compared with the log rank test. Multivariate analysis was done using Cox regression. A p value < 0.05 was considered significant.
Results
Patient’s characteristics and morphology
Eight patients had to be excluded because the FFPE material was insufficient for DNA isolation (< 20% neoplastic cells in the bone marrow) or NGS did not provide reliable results due to reduced DNA quality. Thus, finally, our study group included 30 patients diagnosed with LPL, including 24 cases with WM and 6 with SBCL-PC. Three cases of (IgM) MM were included as a proof of principle. Table 1 summarizes the clinical and Table 2 the pathological characteristics of the final study group.
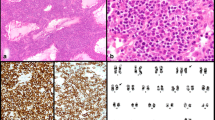
Most lymphomas had a classical WM morphology. Some of them had a more plasmacytoid differentiation and Dutcher bodies were prominent. Immune phenotype was also typical for WM with negativity for CD5 as well as CD23 and variable expression of plasma cell markers (see Fig. 1A–D) in most of the cases.
Classical WM with an admixture of lymphocytes, plasmacytoid lymphocytes and plasma cells (A H&E; inlet highlights plasma cell differentiation; B GIEMSA; plasma cells see arrows); CD20 highlights lymphoma cells within nodular aggregates (C), while plasma cells are located at the edge of the infiltrate (see inlet: immunohistochemistry for vs38c). Plasma cells show clonal restriction by chromogen in situ hybridization (INFORM ISH kappa and lambda, Ventana Medical Systems, Tucson, USA) for the light chains Kappa (D) and Lambda (see inlet) (A, C–D magnification 20 × ; B magnification 40 ×)
The only wild-type WM showed progressive fibrosis and osteosclerosis and had a synchronous diagnosis of diffuse large B-cell-lymphoma (DLBCL) in lung and central nervous system (CNS) (see Fig. 2A–D).
WM wild-type with marked osteosclerosis and fibrosis at diagnosis (A fibrosis is highlighted in the inlet; H&E). The tumor was composed of a mixture of lymphocytes, lymphoplasmacytoid cells, and plasma cells (B; arrows show plasmacytoid cells and plasma cells; H&E). Tumor cells were CD20 positive and partially expressed plasma cell markers (C immunohistochemistry for CD20; inlet: CD138) Plasma cells were clonal (in situ hybridization for light chains kappa and lambda (D Kappa; inlet = Lambda). A DLBCL was diagnosed synchronously in lung and CNS
Two of the six SBCL-PC showed a more variable immune phenotype with additional expression of CD5 and CD23, but also weak or focal expression of plasma cell markers. While one case had polyclonal plasma cells in the background and did not show a MYD88 mutation, the other case was positive for a MYD88 mutation and had monoclonal plasma cells. Both were finally classified as CLL (see suppl. Table 2). Nevertheless, the diagnosis of MYD88 mutations together with clonal plasma cells suggests the existence of a spectrum between B-CLL and classical WM (see Fig. 3A–D).
SBCL-PC finally classified as CLL with features reminiscent of LPL/WM including plasmacytic differentiation with paratrabecular and interstitial infiltrate of lymphocytes (A GIEMSA) admixed with plasma cells (B immunohistochemistry for vs38c), while the majority of the neoplastic cells is CD20 positive (see inlet in B). Additionally, neoplastic cells express CD5 and CD23 (C immunohistochemistry for CD5 and inlet in C immunohistochemistry for CD23). Chromogen in situ hybridization for light chains kappa and lambda showed a clear predominance of kappa positive light chains (D kappa and inlet in D lambda). (all magnification 20 ×)
The other four SBCL-PCs had a more classical WM morphology with paratrabecular and interstitial nodular infiltration (two cases), a striking plasmacytic differentiation (one case) or an interstitial pattern of infiltration being suggestive of a MZL (one case). MMs showed clonal plasma cell proliferations with CD20 and cyclin D1 expression in two of three cases. The best distinguishing features as highlighted in Table 1 for the diagnosis of LPL/WM were morphological parameters, such as infiltration pattern (p = 0.029) and mast cell count (p = 0.001), immune phenotype (p < 0.001) and presence of MYD88 mutation (p < 0.001) (see Table 1).
Next-generation sequencing and its impact on diagnosis
Overall mean number of mutations detected per case was 2 (range 0–12). MYD88 mutations were found in roughly 95% of the cases with WM with CXCR4 mutations being present in 25%. The majority of MYD88 mutations were MYD88 L265P mutations, but in two WM cases, different missense mutations were detected in exon 3 (MYD88 L229S) (see Fig. 1) and exon 1 (MYD88 P34S). Other mutations in WM included mutations of ARID1A (20.8%), KMT2D (12.5%), TP53 (8%), POT1 (8%), TNFAIP3 (8%), and BIRC3 (4%). One case of WM was wild-type with synchronous DLBCL and mutations on CREBBP, EZH2, CARD11, PIM1, SOCS1, STAT6, and TNFRSF14. MYD88 L265P was detected in 50% of SBCL-PC with none of them harboring a CXCR4 mutation or ARID1A mutation. Other mutations in this group included POT1 (4%), MEF2B (4%), FBXW7 (4%), and XPO1 (4%). Taking into account morphology, molecular analysis and clinical characteristics a final diagnosis was achieved in four of six SBCL-PC (2 CLL, one MZL, one WM), while two lymphomas remained unclassifiable (see suppl. Table 2). (IgM) MM cases lacked MYD88 mutation and any other relevant of the analyzed mutations except TP53 in two of three cases (66%). Thus, our results are overall in line with the current literature [1, 10, 14, 21, 23].
Table 3 highlights the type of mutations present in our cases.
To compare molecular analysis with other clinical parameters, we carefully reviewed the literature on known driver mutations in LPL/WM and CLL and we defined molecular groups as follows:
-
Group 1: All patients with MYD88 and/or CXCR4 and/or ARIDA1 mutation without other additional mutations, which are known as driver mutations in lymphomas.
-
Group 2: All patients with MYD88 and/or CXCR4 and/or ARIDA1 mutations plus additional mutations, which are known driver genes in lymphomas (TP53, TNFAIP3, BIRC3, FBXW7, POT1 XPO1).
The three cases of IgM MM were not grouped in either of the categories and thus were not included in further statistics.
When applying the cutoff for mast cell counts as determined by ROC in molecular group 1 significantly more often a mast cell count above the cutoff was observed than in molecular group 2 (17/21 (81%) above the cutoff versus 2/9 (22%) above the cutoff; p = 0.002). The same was observed for immune phenotype (21/21 (100%) versus 7/9 (78%); p = 0.025). However, these results most likely merely depict the fact that group 2 included more cases which were difficult to interpret diagnostically reflected by the immunophenotype and mast cell count.
In seven patients, follow-up biopsies were available for analysis, resulting in 43 finally analyzed bone marrow biopsies. Five of them had progressive disease and MYD88 mutation was the most frequently observed mutation except for one case which had a BIRC3 frameshift mutation in all examined specimens, so far not described in the literature (see Table 5). In four patients with subsequent DLBCL, additional oncogenic mutations were detected in the WM component before the appearance of the aggressive lymphoma component (see Table 4).
Association with disease progression and survival
For analysis of progression-free survival (PFS) and overall survival (OS), we excluded the three MM cases, since these are clinically different and more aggressive entities. In 25 of the remaining 30 patients, information on follow-up concerning disease progression and/or transformation was available.
Table 5 depicts the association of clinical and pathological characteristics with the development of progressive disease, transformation, and death.
Using Kaplan–Meier survival analysis, a significantly better PFS was observed in patients with a paratrabecular and interstitial infiltration pattern compared to those with only interstitial infiltration pattern (8/18 patients, median PFS 112 months versus 5/7patients, median PFS 63 months; p = 0.037, see Fig. 4). None of the other parameters, such as molecular group, age or gender significantly influenced PFS, though a tendency was observed that additional driver mutations lead to a worse PFS (6/17 with progressive disease, median PFS 117.00 months versus 7/8 with progressive disease, median PFS 60.62 months; p = 0.052).
Kaplan–Meier survival analysis for progression-free survival in the 25 patients with WM and SCL-PC: a significantly longer PFS was seen in patients with a paratrabecular and interstitial infiltration pattern compared to those with a purely interstitial pattern (N = number of events/number of patients)
None of the evaluated parameters significantly influenced OS, though interstitial infiltration pattern showed a slight tendency for worse OS (7/18, median OS 140.90 months versus 4/7, median OS 85.67 months; p = 0.097).
Discussion
In our study, we showed that assessment of classical morphological features in the bone marrow, including infiltration pattern and increased numbers of mast cells still are a prerequisite in the diagnosis of WM [26]. In fact, most WMs had higher mast cell counts and infiltration pattern was often (nodular) paratrabecular and interstitial, less likely only interstitial, irrespective if nodular or diffuse–solid [26, 29]. Though presence of MYD88 L265P mutation is helpful in the diagnosis, this mutation is not unique to LPL/WM [1, 30]. Thus, combining careful morphological evaluation with immune phenotyping and molecular analysis in debatable cases is the best way to achieve a proper diagnosis [26, 29]. As already reported in various studies, MYD88 L265P was the most frequently found mutation in LPL/WM also in our study followed by mutations on CXCR4 [1, 15, 23]. In some cases, additional mutations turned up, which are infrequent in LPL/WM. This includes TP53 mutations associated with MYD88 and CXCR4, which were present in 4% (2 cases) of LPL/WM, a finding that is well in line with the literature [19, 31]. Both patients experienced disease progression and finally developed DLBCL with TP53 mutation preceding transformation for 4 and 8 years, respectively (see Table 4). In fact, TP53 mutations predict a worse outcome and rapidly progressive disease [19, 21, 31, 32]. Other mutations include KMT2D missense and frameshift mutations, which appear in a variety of lymphomas. KMT2D are frequently seen in LPLs and mutations have a possible connection to hypogammaglobinemia, autoimmune phenomena and the Kabucki syndrome, but its role in pathogenesis of LPL is still largely unknown [24, 26, 33]. In two cases, TNFAIP3 missense mutations were found, which are associated with a diagnosis of B-cell lymphoma including DLBCL [34, 35]. Functionally TNFAIP3 is a negative regulator in the NFκB pathway and believed to be a tumor suppressor gene [36, 37]. In fact, Compagno et al. [34] who closely investigated DLBCL for the presence and type of TNFAIP3 mutations reported inactivation by a two-hit mechanism such as a combination of inactivating mutations and/or deletions, which is one possible mechanism that promotes lymphomagenesis. Interestingly loss of chromosome 6q, where TNFAIP3 gene is located, is not only a frequent event in DLBCL but is also the main cytogenetic event in WM [32, 34, 37]. Indeed, in one of our two patients with a TNFAIP3 mutation cytogenetics revealed a 6q deletion indicating a similar mechanism. In addition, a TP53 mutation was present and in one patient, who later on developed transformation. Thus, one might speculate that these findings already indicate a more aggressive disease despite morphologic characteristics of WM. Beside one patient diagnosed as LPL/WM was quite interesting due to morphology, molecular genetics, and clinical outcome. Morphologically, a diffuse lymphoplasmacytic infiltrate in the bone marrow with increased mast cells was seen in the bone marrow and immune phenotype was typically for WM. Molecular analysis revealed a MYD88 and a CXCR4 mutation and in addition a frameshift mutation on BIRC3 R434Gfs*12, which was also present in all follow-up biopsies available. Overall tumor burden in the bone marrow remained high (> 40–50%) during the course of disease despite treatment as was allelic burden of BIRC3 and the patient finally succumbed to the disease. BIRC3 mutations appear in up to 4% of newly diagnosed CLL, and rarely in other low-grade lymphomas such as MZL and WM [38]. BIRC3 mutations are mostly nonsense or in-frame variants leading to a loss of function [38, 39]. In brief, degradation of MAPK3K14, which is a major driver of the non-canonical NFκB pathway is induced by BIRC3, thus loss of BIRC3 function constitutively activates the non-canonical pathway resulting in cell proliferation and survival [39]. In CLL, BIRC3 mutations probably indicate a more aggressive course especially in patients treated with fludarabine, cyclophosphamide and rituximab (FCR) [40]. Our case further adds evidence that mutations in BIRC3 might influence treatment response and outcome. Our group included only one MYD88 wild-type LPL/WM, which, in accordance with the literature, had a different molecular profile than those with MYD88 mutations [33]. Furthermore, morphology in the bone marrow showed osteosclerosis and prominent fibrosis features most likely associated with an ongoing transformation to DLBCL, which synchronously occurred in the lung and central nervous system.
In the SBCL-PC group, in addition POT1, FBXW7, XPO1, and MEF2B had mutations. POT1, FBXW7, and XPO1 mutations belong to subgroups of CLL and are known driver mutations [41,42,43,44] but did not occur LPL, so far. However, shared mutations further support the fact that an alliance might exist between these diseases and that mutations are neither specific nor defining for diagnosis. MEF2B mutations present in subgroups of DLBCL and follicular lymphoma most likely act as an oncogene, but LPL or CLL did not show these mutations so far [45, 46]. Functionally, MEF2B is a transcriptional activator leading to enhanced transcription of BCL6 and thus promoting lymphomagenesis [45, 46].
Our study has some limitations. First, the number of cases is small since LPL/WM is a relatively rare disease. Second, the number of progressive and transformed cases is even smaller, since these are even rarer events. Boiza-Sanchez et al. [47] lately reported on the molecular analysis of eight cases of WM with development into a DLBCL, which revealed shared mutations including MYD88 L265P mutation in the majority of cases. We could only analyze the DLBCL component in one patient with bone marrow infiltration, while in all other patients, high-grade lymphoma occurred extra-medullary and FFPE material was not available in sufficient amounts for further analysis. In this patient, the DLBCL component shared mutations with the preceding LPL/WM with an additional EP300 mutation (see Table 4).
To sum up, assessment of classical morphological and immunohistochemical features of WM in the bone marrow is still a prerequisite in the diagnosis of lymphomas with plasmacytic differentiation. The presence of driver mutations in LPL/WM hints at a higher risk of progressive disease and transformation and a shorter PFS, further supporting the usefulness of an in-depth molecular analysis already upon diagnosis or at least in progressive disease to allocate the appropriate treatment. For reasons of cost-efficiency in an increasingly troubled healthcare system stratifying patients for further molecular analysis could be done using conventional parameters, including for example atypical immune phenotype, diffuse infiltration pattern, and reduced mast cell component, and should at least be done in treatment-resistant cases.
Data Availability
The data that support the findings of this study are available from the corresponding author, [A.B.], upon reasonable request.
Abbreviations
- LPL:
-
Lymphoplasmacytic lymphoma
- NGS:
-
Next-generation sequencing
- WM:
-
Waldenstroem’s macroglobulinemia
- SBCL-PC:
-
Small B-cell lymphoma with plasmacytic differentiation
- MM:
-
Multiple myeloma
- MZL:
-
Marginal zone lymphoma
- CLL:
-
Chronic lymphocytic leukemia
- MCL:
-
Mantle cell lymphoma
- BTK:
-
Bruton’s tyrosine kinase
- FFPE:
-
Formalin-fixed paraffin-embedded
- HPF:
-
High-power field
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
References
Wang W, Lin P (2020) Lymphoplasmacytic lymphoma and Waldenstrom macroglobulinaemia: clinicopathological features and differential diagnosis. Pathology 52(1):6–14
Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML et al (2003) Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 30(2):110–115
Gertz MA (2023) Waldenstrom macroglobulinemia: 2023 update on diagnosis, risk stratification, and management. Am J Hematol 98(2):348–358
Naderi N, Yang DT (2013) Lymphoplasmacytic lymphoma and Waldenstrom macroglobulinemia. Arch Pathol Lab Med 137(4):580–585
Kaiser LM, Hunter ZR, Treon SP, Buske C (2021) CXCR4 in Waldenstrom’s macroglobulinema: chances and challenges. Leukemia 35(2):333–345
Garcia-Abellas P, Ferrer Gomez A, Bueno Sacristan D, PirisVillaespesa M, Talavera Yague M, RegueroCallejas ME et al (2022) Lymphoplasmacytic lymphoma and marginal zone lymphoma involving bone marrow: a diagnostic dilemma. Useful clinicopathological features to accurate the diagnosis. EJHaem 3(4):1181–7
Robillard N, Avet-Loiseau H, Garand R, Moreau P, Pineau D, Rapp MJ et al (2003) CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood 102(3):1070–1071
Feyler S, O’Connor SJ, Rawstron AC, Subash C, Ross FM, Pratt G et al (2008) IgM myeloma: a rare entity characterized by a CD20-CD56-CD117- immunophenotype and the t(11;14). Br J Haematol 140(5):547–551
Willenbacher E, Erdel M, Strasser U, Gastl G, Schmidt S, Gunsilius E et al (2008) IgM myeloma: more on a rare entity. Br J Haematol 143(1):146–148
Willenbacher W, Willenbacher E, Brunner A, Manzl C (2013) Improved accuracy of discrimination between IgM multiple myeloma and Waldenstrom macroglobulinaemia by testing for MYD88 L265P mutations. Br J Haematol 161(6):902–904
Abeykoon JP, Paludo J, King RL, Ansell SM, Gertz MA, LaPlant BR et al (2018) MYD88 mutation status does not impact overall survival in Waldenstrom macroglobulinemia. Am J Hematol 93(2):187–194
Zanwar S, Abeykoon JP, Durot E, King R, Perez Burbano GE, Kumar S et al (2020) Impact of MYD88(L265P) mutation status on histological transformation of Waldenstrom macroglobulinemia. Am J Hematol 95(3):274–281
Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R et al (2015) Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med 372(15):1430–1440
Treon SP, Xu L, Guerrera ML, Jimenez C, Hunter ZR, Liu X et al (2020) Genomic landscape of waldenstrom macroglobulinemia and its impact on treatment strategies. J Clin Oncol 38(11):1198–1208
Castillo JJ, Moreno DF, Arbelaez MI, Hunter ZR, Treon SP (2019) CXCR4 mutations affect presentation and outcomes in patients with Waldenstrom macroglobulinemia: a systematic review. Expert Rev Hematol 12(10):873–881
Castillo JJ, Gustine JN, Meid K, Flynn CA, Demos MG, Guerrera ML et al (2020) CXCR4 mutational status does not impact outcomes in patients with Waldenstrom macroglobulinemia treated with proteasome inhibitors. Am J Hematol 95(4):E95–E98
Tam CS, Opat S, D’Sa S, Jurczak W, Lee HP, Cull G et al (2020) A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 136(18):2038–2050
Castillo JJ, Xu L, Gustine JN, Keezer A, Meid K, Dubeau TE et al (2019) CXCR4 mutation subtypes impact response and survival outcomes in patients with Waldenstrom macroglobulinaemia treated with ibrutinib. Br J Haematol 187(3):356–363
Gustine JN, Tsakmaklis N, Demos MG, Kofides A, Chen JG, Liu X et al (2019) TP53 mutations are associated with mutated MYD88 and CXCR4, and confer an adverse outcome in Waldenstrom macroglobulinaemia. Br J Haematol 184(2):242–245
Christian A, Davis Z, Walewska R, McCarthy H (2019) Importance of sequential analysis of TP53 variation in patients with Waldenstrom Macroglobulinaemia. Br J Haematol 186(4):e73–e76
Poulain S, Roumier C, Bertrand E, Renneville A, Caillault-Venet A, Doye E et al (2017) TP53 mutation and its prognostic significance in Waldenstrom’s macroglobulinemia. Clin Cancer Res 23(20):6325–6335
Wang Y, Gali VL, Xu-Monette ZY, Sano D, Thomas SK, Weber DM et al (2021) Molecular and genetic biomarkers implemented from next-generation sequencing provide treatment insights in clinical practice for Waldenström macroglobulinemia. Neoplasia 23(4):361–374
Treon SP, Hunter ZR, Branagan AR, Castillo JJ (2019) Genomic landscape of Waldenstrom’s macroglobulinemia. Hemasphere 3(Suppl):58–61
Varettoni M, Zibellini S, Defrancesco I, Ferretti VV, Rizzo E, Malcovati L et al (2017) Pattern of somatic mutations in patients with Waldenström macroglobulinemia or IgM monoclonal gammopathy of undetermined significance. Haematologica 102(12):2077–2085
Willenbacher E, Willenbacher W, Wolf DG, Zelger B, Peschel I, Manzl C et al (2019) Digital PCR in bone marrow trephine biopsies is highly sensitive for MYD88(L265P) detection in lymphomas with plasmacytic/plasmacytoid differentiation. Br J Haematol 186(1):189–191
Garcia-Reyero J, Martinez Magunacelaya N, Gonzalez de Villambrosia S, Gomez Mediavilla A, Urquieta Lam M, Insunza A et al (2020) Diagnostic value of bone marrow core biopsy patterns in lymphoplasmacytic lymphoma/Waldenström macroglobulinaemia and description of its mutational profiles by targeted NGS. J Clin Pathol 73(9):571–7
Mandrekar JN (2010) Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 5(9):1315–1316
Safari S, Baratloo A, Elfil M, Negida A (2016) Evidence based emergency medicine; part 5 receiver operating curve and area under the curve. Emerg (Tehran) 4(2):111–113
Amaador K, Vos JMI, Pals ST, Kraan W, Dobber JA, Minnema MC et al (2022) Discriminating between Waldenstrom macroglobulinemia and marginal zone lymphoma using logistic LASSO regression. Leuk Lymphoma 63(5):1070–1079
Weber ANR, Cardona Gloria Y, Çınar Ö, Reinhardt HC, Pezzutto A, Wolz OO (2018) Oncogenic MYD88 mutations in lymphoma: novel insights and therapeutic possibilities. Cancer Immunol Immunother 67(11):1797–1807
Krzisch D, Guedes N, Boccon-Gibod C, Baron M, Bravetti C, Davi F et al (2021) Cytogenetic and molecular abnormalities in Waldenström’s macroglobulinemia patients: correlations and prognostic impact. Am J Hematol 96(12):1569–1579
Boutilier AJ, Huang L, Elsawa SF (2022) Waldenström macroglobulinemia: mechanisms of disease progression and current therapies. Int J Mol Sci 23(19):11145
Hunter ZR, Xu L, Tsakmaklis N, Demos MG, Kofides A, Jimenez C et al (2018) Insights into the genomic landscape of MYD88 wild-type Waldenström macroglobulinemia. Blood Adv 2(21):2937–2946
Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q et al (2009) Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 459(7247):717–721
Braggio E, Dogan A, Keats JJ, Chng WJ, Huang G, Matthews JM et al (2012) Genomic analysis of marginal zone and lymphoplasmacytic lymphomas identified common and disease-specific abnormalities. Mod Pathol 25(5):651–660
Poulain S, Braggio E, Roumier C, Aijjou R, Broucqsault N, Galiegue-Zouitina S et al (2011) High-throughput genomic analysis in Waldenstrom’s macroglobulinemia. Clin Lymphoma Myeloma Leuk 11(1):106–108
Wenzl K, Manske MK, Sarangi V, Asmann YW, Greipp PT, Schoon HR et al (2018) Loss of TNFAIP3 enhances MYD88. Blood Cancer J 8(10):97
Onaindia A, Medeiros LJ, Patel KP (2017) Clinical utility of recently identified diagnostic, prognostic, and predictive molecular biomarkers in mature B-cell neoplasms. Mod Pathol 30(10):1338–1366
Tausch E, Stilgenbauer S (2020) mutations in chronic lymphocytic leukemia - uncommon and unfavorable. Haematologica 105(2):255–256
Diop F, Moia R, Favini C, Spaccarotella E, De Paoli L, Bruscaggin A et al (2020) Biological and clinical implications of BIRC3 mutations in chronic lymphocytic leukemia. Haematologica 105(2):448–456
Pérez-Carretero C, Hernández-Sánchez M, González T, Quijada-Álamo M, Martín-Izquierdo M, Hernández-Sánchez JM et al (2020) Chronic lymphocytic leukemia patients with IGH translocations are characterized by a distinct genetic landscape with prognostic implications. Int J Cancer 147(10):2780–2792
Walker JS, Hing ZA, Harrington B, Baumhardt J, Ozer HG, Lehman A et al (2021) Recurrent XPO1 mutations alter pathogenesis of chronic lymphocytic leukemia. J Hematol Oncol 14(1):17
Jain P, Kanagal-Shamanna R, Wierda W, Keating M, Sarwari N, Rozovski U et al (2016) Clinical and molecular characteristics of XPO1 mutations in patients with chronic lymphocytic leukemia. Am J Hematol 91(11):E478–E479
Ramsay AJ, Quesada V, Foronda M, Conde L, Martínez-Trillos A, Villamor N et al (2013) POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat Genet 45(5):526–530
El Jamal SM, Grada Z, El Dinali MH, Zhou H, Hassan SY, Saad AG et al (2019) MEF2B is a member of the BCL6 gene transcriptional complex and induces its expression in diffuse large B-cell lymphoma of the germinal center B-cell-like type. Lab Invest 99(4):539–550
Ying CY, Dominguez-Sola D, Fabi M, Lorenz IC, Hussein S, Bansal M et al (2013) MEF2B mutations lead to deregulated expression of the oncogene BCL6 in diffuse large B cell lymphoma. Nat Immunol 14(10):1084–1092
Boiza-Sánchez M, Manso R, Balagué O, Chamizo C, Askari E, Salgado RN et al (2020) Lymphoplasmacytic lymphoma associated with diffuse large B-cell lymphoma: progression or divergent evolution? PLoS One 15(11):e0241634
Acknowledgements
The authors want to thank Sabine Joebstl for excellent technical assistance.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. This work was funded by the Company Roche.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study on specimens of human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from the ethical committee of the Medical University of Innsbruck (EK-Nr.1362/2020) and informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brunner, A., Thalhammer-Thurner, G.C., Willenbacher, W. et al. In-depth molecular analysis of lymphomas with lymphoplasmacytic differentiation may provide more precise diagnosis and rational treatment allocation. Ann Hematol 103, 553–563 (2024). https://doi.org/10.1007/s00277-023-05531-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05531-9