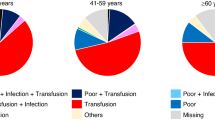
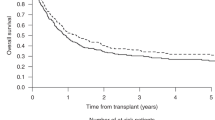
Abstract
Allogeneic hematopoietic stem cell transplantation (allo-SCT) is the sole curative therapy for myelodysplastic syndrome (MDS). However, whether bridging therapy (BRT) including azacitidine (AZA) and combination chemotherapy (CCT) prior to allo-SCT should be performed is unclear. We analyzed BRT and the outcomes of patients with myelodysplastic syndrome with excess blasts (MDS-EB) who were ≤ 70 years old at the time of registration for a prospective observational study to clarify the optimal allo-SCT strategy for high-risk MDS. A total of 371 patients were included in this study. Among 188 patients (50.7%) who were considered for allo-SCT, 141 underwent allo-SCT. Among the patients who underwent allo-SCT, 64 received AZA, 29 received CCT, and 26 underwent allo-SCT without BRT as the initial treatment. Multivariate analysis identified BRT as an independent factor influencing overall survival (AZA vs. without BRT, hazard ratio [HR] 3.33, P = 0.005; CCT vs. without BRT, HR 3.82, P = 0.003). In multivariate analysis, BRT was independently associated with progression-free survival (AZA vs. without BRT: HR, 2.23; P = 0.041; CCT vs. without BRT: HR, 2.94; P = 0.010). Transplant-eligible patients with MDS-EB should undergo allo-SCT when clinically acceptable, and upfront allo-SCT without BRT may be superior to AZA or CCT.




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on a collaborative basis.
References
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C (1982) Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 51(2):189–199
Cazzola M, Malcovati L (2005) Myelodysplastic syndromes–coping with ineffective hematopoiesis. N Engl J Med 352(6):536–538. https://doi.org/10.1056/NEJMp048266
Robin M, Porcher R, Adès L, Raffoux E, Michallet M, François S, Cahn JY, Delmer A, Wattel E, Vigouroux S, Bay JO, Cornillon J, Huynh A, Nguyen S, Rubio MT, Vincent L, Maillard N, Charbonnier A, de Latour RP, Reman O, Dombret H, Fenaux P, Socié G (2015) HLA-matched allogeneic stem cell transplantation improves outcome of higher risk myelodysplastic syndrome A prospective study on behalf of SFGM-TC and GFM. Leukemia 29(7):1496–1501. https://doi.org/10.1038/leu.2015.37
Nakamura R, Saber W, Martens MJ, Ramirez A, Scott B, Oran B, Leifer E, Tamari R, Mishra A, Maziarz RT, McGuirk J, Westervelt P, Vasu S, Patnaik M, Kamble R, Forman SJ, Sekeres MA, Appelbaum F, Mendizabal A, Logan B, Horowitz M, Cutler C (2021) Biologic assignment trial of reduced-intensity hematopoietic cell transplantation based on donor availability in patients 50–75 years of age with advanced myelodysplastic syndrome. J Clin Oncol: Off J Am Soc Clin Oncol 39(30):3328–3339. https://doi.org/10.1200/jco.20.03380
Kröger N, Sockel K, Wolschke C, Bethge W, Schlenk RF, Wolf D, Stadler M, Kobbe G, Wulf G, Bug G, Schäfer-Eckart K, Scheid C, Nolte F, Krönke J, Stelljes M, Beelen D, Heinzelmann M, Haase D, Buchner H, Bleckert G, Giagounidis A, Platzbecker U (2021) Comparison between 5-Azacytidine treatment and allogeneic stem-cell transplantation in elderly patients with advanced MDS according to donor availability (VidazaAllo Study). J Clin Oncol: Off J Am Soc Clin Oncol 39(30):3318–3327. https://doi.org/10.1200/jco.20.02724
Sierra J, Pérez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, Davies SM, Lazarus HM, Bredeson CN, Marks DI, Canals C, Boogaerts MA, Goldman J, Champlin RE, Keating A, Weisdorf DJ, de Witte TM, Horowitz MM (2002) Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood 100(6):1997–2004
Guardiola P, Runde V, Bacigalupo A, Ruutu T, Locatelli F, Boogaerts MA, Pagliuca A, Cornelissen JJ, Schouten HC, Carreras E, Finke J, van Biezen A, Brand R, Niederwieser D, Gluckman E, de Witte TM (2002) Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood 99(12):4370–4378. https://doi.org/10.1182/blood.v99.12.4370
Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL (2012) Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant 18(8):1211–1218. https://doi.org/10.1016/j.bbmt.2012.01.009
Damaj G, Duhamel A, Robin M, Beguin Y, Michallet M, Mohty M, Vigouroux S, Bories P, Garnier A, El Cheikh J, Bulabois CE, Huynh A, Bay JO, Legrand F, Deconinck E, Fegueux N, Clement L, Dauriac C, Maillard N, Cornillon J, Ades L, Guillerm G, Schmidt-Tanguy A, Marjanovic Z, Park S, Rubio MT, Marolleau JP, Garnier F, Fenaux I, Yakoub-Agha I (2012) Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Société Française de Greffe de Moelle et de Thérapie-Cellulaire and the Groupe-Francophone des Myélodysplasies. J Clin Oncol: Off J Am Soc Clin Oncol 30(36):4533–4540. https://doi.org/10.1200/jco.2012.44.3499
Voso MT, Leone G, Piciocchi A, Fianchi L, Santarone S, Candoni A, Criscuolo M, Masciulli A, Cerqui E, Molteni A, Finelli C, Parma M, Poloni A, Carella AM, Spina F, Cortelezzi A, Salvi F, Alessandrino EP, Rambaldi A, Sica S (2017) Feasibility of allogeneic stem-cell transplantation after azacitidine bridge in higher-risk myelodysplastic syndromes and low blast count acute myeloid leukemia: results of the BMT-AZA prospective study. Ann Oncol: Off J Eur Soc Med Oncol 28(7):1547–1553. https://doi.org/10.1093/annonc/mdx154
Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia-Manero G, Malcovati L, Cazzola M, Park S, Itzykson R, Ades L, Fenaux P, Jadersten M, Hellstrom-Lindberg E, Gale RP, Beach CL, Lee SJ, Horowitz MM, Greenberg PL, Tallman MS, DiPersio JF, Bunjes D, Weisdorf DJ, Cutler C (2013) Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol: Off J Am Soc Clin Oncol 31(21):2662–2670. https://doi.org/10.1200/jco.2012.46.8652
Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Pérez WS, Anasetti C, Bolwell BJ, Cairo MS, Gale RP, Klein JP, Lazarus HM, Liesveld JL, McCarthy PL, Milone GA, Rizzo JD, Schultz KR, Trigg ME, Keating A, Weisdorf DJ, Antin JH, Horowitz MM (2004) A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 104(2):579–585. https://doi.org/10.1182/blood-2004-01-0338
Alessandrino EP, Della Porta MG, Pascutto C, Bacigalupo A, Rambaldi A (2013) Should cytoreductive treatment be performed before transplantation in patients with high-risk myelodysplastic syndrome? J Clin Oncol: Off J Am Soc Clin Oncol 31(21):2761–2762. https://doi.org/10.1200/jco.2012.48.0525
Schanz J, Tüchler H, Solé F, Mallo M, Luño E, Cervera J, Granada I, Hildebrandt B, Slovak ML, Ohyashiki K, Steidl C, Fonatsch C, Pfeilstöcker M, Nösslinger T, Valent P, Giagounidis A, Aul C, Lübbert M, Stauder R, Krieger O, Garcia-Manero G, Faderl S, Pierce S, Le Beau MM, Bennett JM, Greenberg P, Germing U, Haase D (2012) New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol: Off J Am Soc Clin Oncol 30(8):820–829. https://doi.org/10.1200/jco.2011.35.6394
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SM, Miyazaki Y, Pfeilstöcker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D (2012) Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120(12):2454–2465. https://doi.org/10.1182/blood-2012-03-420489
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Scott BL, Storer B, Loken MR, Storb R, Appelbaum FR, Deeg HJ (2005) Pretransplantation induction chemotherapy and posttransplantation relapse in patients with advanced myelodysplastic syndrome. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant 11(1):65–73. https://doi.org/10.1016/j.bbmt.2004.10.001
Nakai K, Kanda Y, Fukuhara S, Sakamaki H, Okamoto S, Kodera Y, Tanosaki R, Takahashi S, Matsushima T, Atsuta Y, Hamajima N, Kasai M, Kato S (2005) Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia 19(3):396–401. https://doi.org/10.1038/sj.leu.2403640
Oran B, Kongtim P, Popat U, de Lima M, Jabbour E, Lu X, Chen J, Rondon G, Kebriaei P, Ahmed S, Andersson B, Alousi A, Ciurea S, Shpall E, Champlin RE (2014) Cytogenetics, donor type, and use of hypomethylating agents in myelodysplastic syndrome with allogeneic stem cell transplantation. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant 20(10):1618–1625. https://doi.org/10.1016/j.bbmt.2014.06.022
Onida F, Brand R, van Biezen A, Schaap M, von dem Borne PA, Maertens J, Beelen DW, Carreras E, Alessandrino EP, Volin L, Kuball JH, Figuera A, Sierra J, Finke J, Kröger N, de Witte T (2014) Impact of the International Prognostic Scoring System cytogenetic risk groups on the outcome of patients with primary myelodysplastic syndromes undergoing allogeneic stem cell transplantation from human leukocyte antigen-identical siblings: a retrospective analysis of the European Society for Blood and Marrow Transplantation-Chronic Malignancies Working Party. Haematologica 99(10):1582–1590. https://doi.org/10.3324/haematol.2014.106880
Konuma T, Shimomura Y, Ozawa Y, Ueda Y, Uchida N, Onizuka M, Akiyama M, Mori T, Nakamae H, Ohno Y, Shiratori S, Onishi Y, Kanda Y, Fukuda T, Atsuta Y, Ishiyama K (2019) Induction chemotherapy followed by allogeneic HCT versus upfront allogeneic HCT for advanced myelodysplastic syndrome: A propensity score matched analysis. Hematol Oncol 37(1):85–95. https://doi.org/10.1002/hon.2566
Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan MD, McMichael JF, Wallis JW, Lu C, Shen D, Harris CC, Dooling DJ, Fulton RS, Fulton LL, Chen K, Schmidt H, Kalicki-Veizer J, Magrini VJ, Cook L, McGrath SD, Vickery TL, Wendl MC, Heath S, Watson MA, Link DC, Tomasson MH, Shannon WD, Payton JE, Kulkarni S, Westervelt P, Walter MJ, Graubert TA, Mardis ER, Wilson RK, DiPersio JF (2012) Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481(7382):506–510. https://doi.org/10.1038/nature10738
Parkin B, Ouillette P, Li Y, Keller J, Lam C, Roulston D, Li C, Shedden K, Malek SN (2013) Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood 121(2):369–377. https://doi.org/10.1182/blood-2012-04-427039
Oosterveld M, Suciu S, Muus P, Germing U, Delforge M, Belhabri A, Aul C, Selleslag D, Ferrant A, Marie JP, Amadori S, Jehn U, Mandelli F, Hess U, Hellström-Lindberg E, Cakmak-Wollgast S, Vignetti M, Labar B, Willemze R, de Witte T (2015) Specific scoring systems to predict survival of patients with high-risk myelodysplastic syndrome (MDS) and de novo acute myeloid leukemia (AML) after intensive antileukemic treatment based on results of the EORTC-GIMEMA AML-10 and intergroup CRIANT studies. Ann Hematol 94(1):23–34. https://doi.org/10.1007/s00277-014-2177-y
Schroeder T, Wegener N, Lauseker M, Rautenberg C, Nachtkamp K, Schuler E, Kondakci M, Haas R, Germing U, Kobbe G (2019) Comparison between Upfront Transplantation and different Pretransplant Cytoreductive Treatment Approaches in Patients with High-Risk Myelodysplastic Syndrome and Secondary Acute Myelogenous Leukemia. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant 25(8):1550–1559. https://doi.org/10.1016/j.bbmt.2019.03.011
Rautenberg C, Germing U, Stepanow S, Lauseker M, Köhrer K, Jäger PS, Geyh S, Fan M, Haas R, Kobbe G, Schroeder T (2021) Influence of somatic mutations and pretransplant strategies in patients allografted for myelodysplastic syndrome or secondary acute myeloid leukemia. Am J Hematol 96(1):E15-e17. https://doi.org/10.1002/ajh.26013
Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, Grauman PV, Hu ZH, Spellman SR, Lee SJ, Verneris MR, Hsu K, Fleischhauer K, Cutler C, Antin JH, Neuberg D, Ebert BL (2017) Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med 376(6):536–547. https://doi.org/10.1056/NEJMoa1611604
Della Porta MG, Gallì A, Bacigalupo A, Zibellini S, Bernardi M, Rizzo E, Allione B, van Lint MT, Pioltelli P, Marenco P, Bosi A, Voso MT, Sica S, Cuzzola M, Angelucci E, Rossi M, Ubezio M, Malovini A, Limongelli I, Ferretti VV, Spinelli O, Tresoldi C, Pozzi S, Luchetti S, Pezzetti L, Catricalà S, Milanesi C, Riva A, Bruno B, Ciceri F, Bonifazi F, Bellazzi R, Papaemmanuil E, Santoro A, Alessandrino EP, Rambaldi A, Cazzola M (2016) Clinical effects of driver somatic mutations on the outcomes of patients with myelodysplastic syndromes treated with allogeneic hematopoietic stem-cell transplantation. J Clin Oncol: Off J Am Soc Clin Oncol 34(30):3627–3637. https://doi.org/10.1200/jco.2016.67.3616
Yoshizato T, Nannya Y, Atsuta Y, Shiozawa Y, Iijima-Yamashita Y, Yoshida K, Shiraishi Y, Suzuki H, Nagata Y, Sato Y, Kakiuchi N, Matsuo K, Onizuka M, Kataoka K, Chiba K, Tanaka H, Ueno H, Nakagawa MM, Przychodzen B, Haferlach C, Kern W, Aoki K, Itonaga H, Kanda Y, Sekeres MA, Maciejewski JP, Haferlach T, Miyazaki Y, Horibe K, Sanada M, Miyano S, Makishima H, Ogawa S (2017) Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: impact on outcome of stem cell transplantation. Blood 129(17):2347–2358. https://doi.org/10.1182/blood-2016-12-754796
Acknowledgements
We appreciate all physicians and staff participating in the JALSG-CS11-MDS-SCT study. We are grateful to the JALSG staff.
Funding
Funded by Japan Adult Leukemia Study Group (JALSG).
Author information
Authors and Affiliations
Contributions
Noriharu Nakagawa, Ken Ishiyama, Kensuke Usuki, Shigeki Ohtake, Akira Katsumi, Hitoshi Kiyoi, Itaru Matsumura, and Yasushi Miyazaki designed the study. Satoru Takada, Tatsuki Tomikawa, Hiroshi Handa, Yuna Katsuoka, Daiki Hirano, Nobuo Sezaki, Masahiko Sumi, Shin Fujisawa, Yasuhiro Taniguchi, Atsuko Mugitani, Takuro Yoshimura, Eiichi Ohtsuka, Ken Takase, Youko Suehiro, Shuichi Ota, Tomohiro Kajiguchi, Tomoya Maeda and Masahide Yamamoto collected clinical data. Noriharu Nakagawa and Ken Ishiyama analyzed the data and wrote the manuscript. All the authors critically reviewed the manuscript and checked the final version.
Corresponding author
Ethics declarations
Ethical approval
This study was approved as an accompanying study of JALSG-CS-11 by the committee for the Japan Adult Leukemia Study Group (JALSG) and by the ethical committee of Kanazawa University (No. 2018–227) and all participating institutions. Informed consent was obtained from all the participants.
Conflict of interest
Kensuke Usuki has received research funding from Astellas-Amgen-Biopharma, Otsuka, Apellis, SymBio, Takeda, Nippon Shinyaku, Novartis, AbbVie, Janssen, Bristol-Myers Squibb, Ono, Chugai, Daiichi Sankyo, MSD, Astellas, Alexion, Kyowa-Kirin, Gilead, Pfizer, Incyte, SymBio, Celgene, Sumitomo-Dainippon, Mundi, Yakult, and Eisai, and has served on speaker bureaus for Novartis, Astellas, Alexion, Eisai, MSD, Otsuka, Ono, Kyowa-Kirin, Celgene, Daiichi Sankyo, Takeda, Nippon-Shinyaku, PharmaEssentia, Bristol-Myers Squibb, Yakult, Sanofi, Pfizer, AbbVie, and Chugai, and has served on consulting bureaus for Alexion, SymBio, Nippon Shinyaku, Otsuka, Chugai, Sanofi, Takeda, Kyowa-Kirin, Astellas, SOBI, and Alnylam Japan. Shin Fujisawa received honoraria from Bristol-Myers-Squibb, Astellas, Nippon Shinyaku, Otsuka, Pfizer, Novartis, MSD, Sanofi, Janssen, SymBio, Kyowa Hakko Kirin, AstraZeneca, CSL Behring, Meiji Seika Pharma, AbbVie, Takeda, and Chugai, and received research funding from Shionogi, Kyowa Hakko Kirin, Chugai, Otsuka, Asahi-Kasei, and Daiichi Sankyo. Tomoya Maeda received honoraria from Amgen, Nippon Becton Dickinson Company, Nippon Shinyaku, Novartis, Otsuka, Pfizer, and TOPPAN and received research funding from Chugai, Eisai, and Sumitomo Pharma. Masahide Yamamoto received honoraria from Bristol-Myers Squibb, Chugai, Eisai, Kyowa-Kirin, Nippon Shinyaku, Novartis, Ono, Otsuka, Pfizer, Takeda, Sanofi, Janssen, AbbVie, SymBio, and Meiji Seika. Hitoshi Kiyoi received research funding from FUJIFILM, Kyowa-Kirin, Bristol-Myers Squibb, Otsuka, Perseus Proteomics, Daiichi Sankyo, AbbVie, CURED, Astellas, Chugai, Zenyaku Kogyo, Nippon Shinyaku, Eisai, Takeda, Sumitomo Pharma, Sanofi, and Honoraria from AbbVie, Chugai, Astellas Pharma, and Novartis. Itaru Matsumura has received research funding from Kyowa-Kirin, Chugai, Novartis, Astellas, AbbVie, Pfizer, Takeda, Alexion, Otsuka, Janssen, Sumitomo-Dainippon, Shionogi, Asahi-Kasei, Eisai, TAIHO, Nippon Shinyaku, Ono, Sanofi, and Mitsubashi Tanabe and has received honoraria from Novartis, Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, Otsuka, Astellas, Janssen, AbbVie, Takeda, Ono, and SymBio. Yasushi Miyazaki received honoraria from Nippon Shinyaku, Bristol-Myers Squib, Novartis, Sumitomo Pharma, Kyowa-Kirin, AbbVie, Daiichi-Sankyo, Takeda, Janssen Pharmaceutical, Astellas, Pfizer, Chugai, SymBio, and Otsuka Pharmaceutical, and research funding from Sumitomo-Dainippon.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplemental figure 1.
The survival of patients in the SCT-considered cohort with blasts ≥10% at the diagnosis. A. The overall survival of patients treated with each BRT. B. The overall survival of patients with each BRT adjusted for background factors, including age, sex, PS at diagnosis, cytogenetic risk, and allo-SCT (PNG 143 kb)

Supplemental figure 2.
The survival of patients in the transplant cohort. A. The overall survival according to sex. B: The overall survival according to cytogenetic risk. C. The overall survival according to sex and cytogenetic risk (PNG 209 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakagawa, N., Ishiyama, K., Usuki, K. et al. Outcomes of transplant-eligible patients with myelodysplastic syndrome with excess blasts registered in an observational study: The JALSG-CS11-MDS-SCT. Ann Hematol 103, 307–320 (2024). https://doi.org/10.1007/s00277-023-05527-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05527-5