Abstract
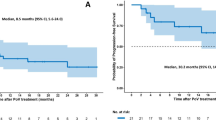
Relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) is a challenging condition to treat, and there is an unmet clinical need for effective therapies. Recently, polatuzumab vedotin (Pola), an anti-CD79b antibody-drug-conjugate (ADC), combined with bendamustine-rituximab (BR), has been approved for R/R DLBCL patients. However, real-world data on Pola-based regimens in R/R DLBCL patients, especially in Thailand, are limited. This study aimed to evaluate the efficacy and safety of Pola-based salvage treatment in R/R DLBCL patients in Thailand. Thirty-five patients who received Pola-based treatment were included in the study, and their data were compared to 180 matched patients who received non-Pola-based therapy. The overall response rate (ORR) in the Pola group was 62.8%, with complete remission and partial remission rates of 17.1% and 45.7%, respectively. The median progression-free survival (PFS) and overall survival (OS) were 10.6 months and 12.8 months, respectively. The study found a significantly higher ORR in Pola-based salvage treatments compared to non-Pola-based therapy (62.8% vs. 33.3%). The survival outcomes were also significantly superior in the Pola group, with longer median PFS and OS than the control group. Grades 3–4 adverse events (AEs) were mainly hematological, and they were tolerable. In conclusion, this study provides real-world evidence of the efficacy and safety of Pola-based salvage treatment in R/R DLBCL patients in Thailand. The results of this study are promising and suggest that Pola-based salvage treatment could be a viable option for R/R DLBCL patients who have limited treatment options.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
National Cancer Institute (2020) Cancer stat facts: diffuse large B-cell lymphoma. https://seer.cancer.gov/statfacts/html/dlbcl.html. Accessed 9 Nov 2022
Sant M, Allemani C, Tereanu C et al (2010) Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 116(19):3724–3734
Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS (2015) Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol 90(9):790–795
Intragumtornchai T, Bunworasate U, Wudhikarn K et al (2018) Non-Hodgkin lymphoma in South East Asia: an analysis of the histopathology, clinical features, and survival from Thailand. Hematol Oncol 36(1):28–36
Coiffier B, Lepage E, Briere J et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346(4):235–242
Pfreundschuh M, Kuhnt E, Trumper L et al (2011) CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 12(11):1013–1022
International Non-Hodgkin’s Lymphoma Prognostic Factors P (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 329(14):987–994
Alizadeh AA, Eisen MB, Davis RE et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403(6769):503–511
Rosenwald A, Wright G, Chan WC et al (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346(25):1937–1947
Shipp MA, Ross KN, Tamayo P et al (2002) Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med 8(1):68–74
Sehn LH, Berry B, Chhanabhai M et al (2007) The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109(5):1857–1861
Crump M, Neelapu SS, Farooq U et al (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 130(16):1800–1808
Vitolo U, Trneny M, Belada D et al (2017) Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol 35(31):3529–3537
Schmitz R, Wright GW, Huang DW et al (2018) Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 378(15):1396–1407
Gisselbrecht C, Glass B, Mounier N et al (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28(27):4184–4190
Gisselbrecht C, Schmitz N, Mounier N et al (2012) Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol 30(36):4462–4469
Nagle SJ, Woo K, Schuster SJ et al (2013) Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol 88(10):890–894
Hamadani M, Hari PN, Zhang Y et al (2014) Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 20(11):1729–1736
Crump M (2016) Management of relapsed diffuse large B-cell lymphoma. Hematol Oncol Clin North Am 30(6):1195–1213
Van Den Neste E, Schmitz N, Mounier N et al (2016) Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant 51(1):51–57
Locke FL, Ghobadi A, Jacobson CA et al (2019) Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 20(1):31–42
Abramson JS, Palomba ML, Gordon LI et al (2020) Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396(10254):839–852
Schuster SJ, Tam CS, Borchmann P et al (2021) Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 22(10):1403–1415
Westin JR, Kersten MJ, Salles G et al (2021) Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am J Hematol 96(10):1295–1312
The NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines®) for Diffuse large B-cell lymphoma version 5.2022 (2022) © National Comprehensive Cancer Network, Inc.
Dornan D, Bennett F, Chen Y et al (2009) Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 114(13):2721–2729
Palanca-Wessels MC, Czuczman M, Salles G et al (2015) Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol 16(6):704–715
Morschhauser F, Flinn IW, Advani R et al (2019) Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol 6(5):e254–e265
Sehn LH, Herrera AF, Flowers CR et al (2020) Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 38(2):155–165
Sehn LH, Hertzberg M, Opat S et al (2022) Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv 6(2):533–543
Alaggio R, Amador C, Anagnostopoulos I et al (2022) The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia 36(7):1720–1748
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A (2021) Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 112(1):90–92
Wang H, Chow S-C (2014) Sample size calculation for comparing proportions. Wiley StatsRef: Statistics Reference online pp 1–16
Dimou M, Papageorgiou SG, Stavroyianni N et al (2021) Real-life experience with the combination of polatuzumab vedotin, rituximab, and bendamustine in aggressive B-cell lymphomas. Hematol Oncol 39(3):336–348
Liebers N, Duell J, Fitzgerald D et al (2021) Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas. Blood Adv 5(13):2707–2716
Segman Y, Ribakovsky E, Avigdor A et al (2021) Outcome of relapsed/refractory diffuse large B-cell lymphoma patients treated with polatuzumab vedotin-based therapy: real-life experience. Leuk Lymphoma 62(1):118–124
Smith SD, Lopedote P, Samara Y et al (2021) Polatuzumab vedotin for relapsed/refractory aggressive B-cell lymphoma: a multicenter post-marketing analysis. Clin Lymphoma Myeloma Leuk 21(3):170–175
Northend M, Wilson W, Osborne W et al (2022) Results of a United Kingdom real-world study of polatuzumab vedotin, bendamustine, and rituximab for relapsed/refractory DLBCL. Blood Adv 6(9):2920–2926
Vodicka P, Benesova K, Janikova A et al (2022) Polatuzumab vedotin plus bendamustine and rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma in the real world. Eur J Haematol 109(2):162–165
Terui Y, Rai S, Izutsu K et al (2021) A phase 2 study of polatuzumab vedotin + bendamustine + rituximab in relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci 112(7):2845–2854
Tilly H, Morschhauser F, Sehn LH et al (2022) Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med 386(4):351–363
Song Y, Tilly H, Rai S et al (2023) Polatuzumab vedotin in previously untreated DLBCL: an Asia subpopulation analysis from the phase 3 POLARIX trial. Blood 141(16):1971–1981
Acknowledgements
The authors thank the collaboration of Thai lymphoma study group (TLSG) members on behalf of the Thai Society of Hematology (TSH), who contributed to the patient care in this study.
Author information
Authors and Affiliations
Contributions
Thanawat Rattanathammethee and Lalita Norasetthada contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Thanawat Rattanathammethee. The first draft of the manuscript was written by Thanawat Rattanathammethee. Tanin Intragumtornchai and Kitsada Wudhikarn commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the International Conference on Harmonization for Good Clinical Practice guidelines and the 1964 Declaration of Helsinki. The study was approved by the ethic committees of all the participating sites (Study code: MED-2564–08034). The informed consent was exempted due to the retrospective cohort study design.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rattanathammethee, T., Norasetthada, L., Bunworasate, U. et al. Outcomes of polatuzumab vedotin-containing regimens in real-world setting of relapsed and or refractory diffuse large B-cell lymphoma patients: a matched-control analysis from the Thai Lymphoma Study Group (TLSG). Ann Hematol 102, 1887–1895 (2023). https://doi.org/10.1007/s00277-023-05273-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05273-8