Abstract
Intensive chemotherapy is the backbone of induction treatment in patients with acute myeloid leukemia (AML). However, AML patients with concomitant cardiac disease may not be eligible for anthracycline-based therapies. In a small cohort of patients, we have previously shown that anthracycline-free, amsacrine-based chemotherapy TAA (thioguanine, cytarabine, amsacrine) may be as effective as cytarabine/daunorubicin for induction therapy in these patients. In this systematic retrospective single-center analysis, we documented the outcome of 31 patients with significant cardiac comorbidities including coronary heart disease or cardiomyopathy receiving TAA as induction chemotherapy. Median (range) ejection fraction (EF) was 48% (30–67%) in this cohort. Patients with EF below 30% were considered unfit for intensive induction therapy. Event-free survival (EFS), overall survival (OS), and relapse-free survival (RFS) were 1.61, 5.46, and 13.6 months respectively. Poor outcome was primarily related to a high early mortality rate within the first 30 days of therapy, mainly caused by infectious complications. TAA cannot be recommended as a substitute of standard induction for AML patients with significant concomitant cardiac disease. In the era of novel agents, alternative strategies (e.g., hypomethylating agents plus venetoclax) should be considered when anthracycline-based regimens are not suitable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a disease of the elderly population with a median age of 72 years at diagnosis [1, 2]. Outcome in older patients is poor compared to younger patients, mainly due to higher early mortality and lower remission rates [3]. In addition to adverse genetic and other biological disease characteristics with impact on prognosis in elderly AML, advanced age often entails a higher likelihood for comorbidities that may preclude standard induction chemotherapy [4]. Anthracycline-based chemotherapy is the backbone of induction therapy in AML with the intention to achieve first complete remission before subsequent consolidation treatment with either conventional chemotherapy or hematopoietic stem cell transplantation (HSCT). Due to the high prevalence of concomitant cardiac disease in elderly patients, it can be challenging for clinicians to identify an appropriate induction regimen [5, 6].
Several recommendations have been made for the treatment of AML patients with significant cardiac comorbidities. As long as the ejection fraction (EF) has not fallen below 45%, patients may be treated with standard induction therapy [7]. The risk of clinical heart failure can be decreased by reducing the anthracycline dose or prolonging the anthracycline infusion time without significantly compromising disease control [8, 9]. The use of cardioprotective drugs and optimized supportive therapy may further reduce the risk of complications during intensive induction therapy [10, 11]. However, patients with coronary heart disease or patients after myocardial infarction do not necessarily suffer from reduced EF and even after optimization of cardiac therapy, they remain at high risk for complications [8, 9].
Recently, various novel approaches have emerged based on the lower-intensity treatment backbones azacitidine and decitabine (hypomethylating agents (HMA)) or low-dose cytarabine (LDAC). These include the B-cell lymphoma 2 (BCL2) inhibitor venetoclax, the hedgehog inhibitor glasdegib, as well as FMS-like tyrosine kinase 3 (FLT3)- and isocitrate dehydrogenase (IDH) 1/2-inhibitors [12]. Some of these regimens have shown impressive response rates with comparably moderate toxicity [13,14,15,16,17,18,19,20,21,22]. Especially in patients with significant comorbidities, these regimens provide attractive alternatives to standard induction chemotherapies.
In the past, intensive anthracycline-free chemotherapy regimens have been proposed for patients who are considered fit for intensive therapy in principle but show significant cardiac comorbidities [7, 23, 24]. In this context, others and we have reported that replacement of anthracyclines by amsacrine may provide an alternative intensive induction regimen with less cardiac toxicity and without compromising complete remission rates in AML [25,26,27,28,29,30,31]. Thus, amsacrine-based induction has been repeatedly used in the past decades in AML patients with cardiac comorbidities but otherwise preserved performance status.
In this retrospective single-center analysis, we re-evaluated outcomes of patients receiving the TAA regimen as induction chemotherapy in a comparatively large cohort of intensively treated AML patients with cardiac comorbidities between 2009 and 2020.
Data and statistical analysis
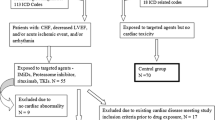
Patients with newly diagnosed de novo, secondary, or therapy-related AML (other than acute promyelocytic leukemia) were included. Patients with cardiac comorbidities, including cardiomyopathy, cardiovascular disease, or heart failure, who were not eligible for treatment with cardiotoxic anthracyclines were considered for treatment with amsacrine-based induction chemotherapy TAA (200 mg/m2 thioguanine on days 3–9, 210 mg/m2 amsacrine on days 3–5 and 200 mg/m2 cytarabine over 24 h on days 1–8). Decision for induction therapy was made in the hematological tumor board of our center.
Remission status was defined according to European LeukemiaNet (ELN) criteria [32]. Patients achieving complete remission (CR) upon induction therapy received consolidation treatment with either 2 or 3 courses of intermediate/high-dose cytarabine (IDAC/HDAC) or allogeneic hematopoietic stem cell transplantation (HSCT). IDAC was dosed at 1 g/m2 (every 12 h days 1, 3, and 5) except in one patient who received doses of 3 g/m2 (HDAC).
Cardiac function of each patient was assessed via transthoracic echocardiography before start of therapy. If only qualitative results were available, numeric equivalents were chosen according to the guidelines of the British Society of Echocardiography [33].
Survival times were plotted using Kaplan–Meier curves. Relapse-free survival (RFS) was calculated from the time of CR/CRi until relapse or death. Event-free survival (EFS) was calculated from start of induction therapy until induction failure, relapse after CR/CRi or death, whichever occurred first. We performed multivariable Cox regression analysis to evaluate the effects of different variables on OS. Likewise, multi-variable logistic regression models were computed to assess the influence of sex, age, EF, WBC, LDH, blast count, AML type, and genetic risk according to the ELN2010 classification on early death events. p values < 0.05 were considered to indicate significant differences. Calculations and plots were performed using the statistical environment R (version 4.2.0).
Results
Between 2009 and 2020, a total of 31 patients were treated with TAA induction at our department. Patient characteristics are summarized in Table 1. Median (range) age of all patients was 63 (38 –77) years. The most common concomitant cardiac disease was coronary artery disease, which was found in 15 (48%) of these patients, followed by cardiomyopathy in 6 patients (19%) and chronic heart failure, which occurred in 4 patients (13%). The remaining 6 patients suffered from various concomitant cardiac diseases such as atrial fibrillation or severe aortic valve stenosis. If patients had more than one heart condition, they were classified according to the most severe or clinically leading condition. Median (range) EF was 48% (30–67%). Reviewing the medical records of all patients and identified 9 patients with additional noncardiac comorbidities: three patients with type II diabetes, two patients with chronic kidney disease, three patients with a history of stroke or cerebral haemorrhage, and one patient with rheumatoid arthritis. After the first cycle of induction therapy, 13 patients (42%) in this cohort had no residual blasts (< 5%) on a day 15 bone marrow evaluation. Eight (26%) patients did not receive a bone marrow aspirate on day 15, either due to early death or poor general performance status. A second cycle of induction therapy was given in 5 (16.1%) cases. Following one or two induction cycles, 16 patients (52%) achieved CR/CRi.
Median EFS was 1.61 months (95% CI 0.72–13.3) (Fig. 1a). Median OS was 5.46 months (95% CI 0.82–17.7) (Fig. 1b). Early mortality within the first 30 days of induction was high, accounting for 12 fatalities (39%). Main cause of death were infectious complications (Table 2). In the 16 patients achieving a CR/CRi, median RFS was 13.6 months (95% CI 11.3–NA) (Fig. 1c).
Of the 31 patients treated with TAA, 10 (32%) patients received an allogeneic HSCT. Median overall survival landmarked from the date of transplantation was 51.4 (95% CI 5.52–NA) months (Fig. 1d).
In multivariable Cox regression analyses, age and genetic risk were significantly associated with OS (HR 1.1 CI 1.02–1.18, p = 0.012; HR 0.09 CI 0.01 – 0.85, p = 0.035; Table 3). A higher blast count at diagnosis was significantly associated with increased risk of early death in a binary logistic regression analysis (p = 0.038).
Discussion
In this systematic retrospective single-center analysis, median OS (5.46 months) and 5-year survival (< 10%) following induction therapy with TAA were dismal, mainly because of the high early-death rate related to infectious complications. Our findings are based on a patient cohort treated between 2009 and 2020 and contrast with a former analysis from our department where the TAA induction therapy applied to patients between 1997 and 2003 has been shown to be equally tolerated and efficacious compared to 7 + 3 induction [25]. This discrepancy may result from a smaller sample size of the previous study and the continuous improvement of supportive care strategies since 2008, leading to more patients being referred to intensive treatment despite of comparatively higher comorbidities. Consequently, risk of early death may have increased in our vulnerable cohorts of AML patients over time.
The high early death rate in our analysis exceeds most published rates for intensive AML induction regimens in a cohort of this age [2, 34,35,36]. Previous studies indicated that amsacrine-based and other common intensive induction regimens are equivalent in AML therapy in terms of toxicity and early death. However, these studies did not necessarily include patients with severe comorbidities [26,27,28,29,30,31]. Our data suggests unacceptably high treatment-related toxicity of the TAA regimen in AML patients with significant cardiac diseases who were otherwise considered eligible for intensive treatment.
EFS was reduced in our analysis compared with published data, whereas RFS was comparable to published results after induction therapy, underlining the antileukemic efficacy of the TAA regimen [37, 38].
Although our analysis suffers from the limitations of a single-center retrospective study, our results strongly imply that patients with significant concomitant cardiac disease should not be treated with amsacrine-based induction chemotherapy. We believe that whenever application of an anthracycline-based induction regimen is contraindicated, alternative substances should be preferred, e.g., HMA or other targeted therapies. It should be noted that also venetoclax-based therapies may be associated with cardiac complications, even in the absence of pre-existing cardiac disease [39]. However, in terms of response rates, these alternative therapies have been shown to be very effective in AML and do not preclude consolidation with allogeneic HSCT with curative intent [40,41,42,43].
Data availability
The original data are available by e-mail from the corresponding author on reasonable request and provision of data must be in compliance with the relevant legal regulations.
References
Shallis RM, Wang R, Davidoff A et al (2019) Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev 36:70–87. https://doi.org/10.1016/j.blre.2019.04.005
Juliusson G, Antunovic P, Derolf Å et al (2009) Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 113:4179–4187. https://doi.org/10.1182/blood-2008-07-172007
Appelbaum FR, Gundacker H, Head DR et al (2006) Age and acute myeloid leukemia. Blood 107:3481–3485. https://doi.org/10.1182/blood-2005-09-3724
Solomon SR, Solh M, Jackson KC et al (2020) Real-world outcomes of unselected elderly acute myeloid leukemia patients referred to a leukemia/hematopoietic cell transplant program. Bone Marrow Transplant 55:189–198. https://doi.org/10.1038/s41409-019-0675-1
Østgård LSG, Nørgaard JM, Sengeløv H et al (2015) Comorbidity and performance status in acute myeloid leukemia patients: a nation-wide population-based cohort study. Leukemia 29:548–555. https://doi.org/10.1038/leu.2014.234
Dhopeshwarkar N, Iqbal S, Wang X, Salas M (2019) A retrospective study of comorbidities and complications in elderly acute myeloid leukemia patients in the United States. Clin Lymphoma Myeloma Leuk 19:e436–e456. https://doi.org/10.1016/j.clml.2019.04.012
Ofran Y, Tallman MS, Rowe JM (2016) How I treat acute myeloid leukemia presenting with preexisting comorbidities. Blood 128:488–496. https://doi.org/10.1182/blood-2016-01-635060
Dalen EC, Pal HJ, Kremer LC (2016) Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev 2016. https://doi.org/10.1002/14651858.CD005008.pub4
Burnett AK, Russell NH, Hills RK et al (2015) A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 125:3878–3885. https://doi.org/10.1182/blood-2015-01-623447
Bosch X, Rovira M, Sitges M et al (2013) Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 61:2355–2362. https://doi.org/10.1016/j.jacc.2013.02.072
Georgakopoulos P, Roussou P, Matsakas E et al (2010) Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol 85:894–896. https://doi.org/10.1002/ajh.21840
Döhner H, Wei AH, Löwenberg B (2021) Towards precision medicine for AML. Nat Rev Clin Oncol 18:577–590. https://doi.org/10.1038/s41571-021-00509-w
DiNardo CD, Pratz K, Pullarkat V et al (2019) Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133:7–17. https://doi.org/10.1182/blood-2018-08-868752
Wei AH, Strickland SA, Hou J-Z et al (2019) Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. JCO 37:1277–1284. https://doi.org/10.1200/JCO.18.01600
DiNardo CD, Schuh AC, Stein EM et al (2019) Enasidenib plus azacitidine significantly improves complete remission and overall response compared with azacitidine alone in patients with newly diagnosed acute myeloid leukemia (AML) with isocitrate dehydrogenase 2 (IDH2) mutations: interim phase II results from an ongoing, randomized study. Blood 134:643–643. https://doi.org/10.1182/blood-2019-130362
DiNardo CD, Stein AS, Stein EM et al (2020) Mutant isocitrate dehydrogenase 1 inhibitor ivosidenib in combination with azacitidine for newly diagnosed acute myeloid leukemia. J Clin Oncol JCO2001632. https://doi.org/10.1200/JCO.20.01632
Cortes JE, Heidel FH, Hellmann A et al (2019) Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 33:379–389. https://doi.org/10.1038/s41375-018-0312-9
Zeidan AM, Schuster MW, Krauter J et al (2019) Clinical benefit of glasdegib in combination with azacitidine or low-dose cytarabine in patients with acute myeloid leukemia. Blood 134:3916–3916. https://doi.org/10.1182/blood-2019-124034
Perl AE, Martinelli G, Cortes JE et al (2019) Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 381:1728–1740. https://doi.org/10.1056/NEJMoa1902688
Heuser M, Smith BD, Fiedler W et al (2021) Clinical benefit of glasdegib plus low-dose cytarabine in patients with de novo and secondary acute myeloid leukemia: long-term analysis of a phase II randomized trial. Ann Hematol 100:1181–1194. https://doi.org/10.1007/s00277-021-04465-4
Cortes JE, Heidel FH, Fiedler W et al (2020) Survival outcomes and clinical benefit in patients with acute myeloid leukemia treated with glasdegib and low-dose cytarabine according to response to therapy. J Hematol Oncol 13:92. https://doi.org/10.1186/s13045-020-00929-8
Stein EM, DiNardo CD, Fathi AT et al (2021) Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: a phase 1 study. Blood 137:1792–1803. https://doi.org/10.1182/blood.2020007233
Bashey A, Liu L, Ihasz A et al (2006) Non-anthracycline based remission induction therapy for newly diagnosed patients with acute myeloid leukemia aged 60 or older. Leuk Res 30:503–506. https://doi.org/10.1016/j.leukres.2005.09.002
DiNardo CD, Wei AH (2020) How I treat acute myeloid leukemia in the era of new drugs. Blood 135:85–96. https://doi.org/10.1182/blood.2019001239
Kessler T, Mohr M, Müller-Tidow C et al (2008) Amsacrine containing induction therapy in elderly AML patients: comparison to standard induction regimens in a matched-pair analysis. Leuk Res 32:491–494. https://doi.org/10.1016/j.leukres.2007.06.015
Arlin ZA, Feldman EJ, Mittelman A et al (1991) Amsacrine is safe and effective therapy for patients with myocardial dysfunction and acute leukemia. Cancer 68:1198–1200. https://doi.org/10.1002/1097-0142(19910915)68:6%3c1198::AID-CNCR2820680603%3e3.0.CO;2-E
Brandwein JM, Geddes M, Kassis J et al (2013) Treatment of older patients with acute myeloid leukemia (AML): a Canadian consensus. Am J Blood Res 3:141–164
Fong CY, Grigoriadis G, Hocking J et al (2013) Fludarabine, cytarabine, granulocyte-colony stimulating factor and amsacrine: an effective salvage therapy option for acute myeloid leukemia at first relapse. Leuk Lymphoma 54:336–341. https://doi.org/10.3109/10428194.2012.713479
Pfrepper C, Klink A, Behre G et al (2016) Risk factors for outcome in refractory acute myeloid leukemia patients treated with a combination of fludarabine, cytarabine, and amsacrine followed by a reduced-intensity conditioning and allogeneic stem cell transplantation. J Cancer Res Clin Oncol 142:317–324. https://doi.org/10.1007/s00432-015-2050-y
Linkesch W, Michlmayr G, Gerhartz H et al (1989) Amsacrine, cytarabine and thioguanine (AAT) versus daunorubicin, cytarabine, thioguanine (DAT) in adults with untreated acute non-lymphoblastic leukemia (ANLL). Austrian-German results Onkologie 12:8–10. https://doi.org/10.1159/000216587
Berman E, Arlin ZA, Gaynor J et al (1989) Comparative trial of cytarabine and thioguanine in combination with amsacrine or daunorubicin in patients with untreated acute nonlymphocytic leukemia: results of the L-16M protocol. Leukemia 3:115–121
Döhner H, Estey E, Grimwade D et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424–447. https://doi.org/10.1182/blood-2016-08-733196
Harkness A, Ring L, Augustine DX et al (2020) Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: a guideline from the British Society of Echocardiography. Echo Res Pract 7:G1–G18. https://doi.org/10.1530/ERP-19-0050
Liu C-J, Hong Y-C, Kuan AS et al (2020) The risk of early mortality in elderly patients with newly diagnosed acute myeloid leukemia. Cancer Med 9:1572–1580. https://doi.org/10.1002/cam4.2740
Hahn A, Giri S, Yaghmour G, Martin MG (2015) Early mortality in acute myeloid leukemia. Leuk Res 39:505–509. https://doi.org/10.1016/j.leukres.2015.02.003
Löwenberg B, Ossenkoppele GJ, van Putten W et al (2009) High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 361:1235–1248. https://doi.org/10.1056/NEJMoa0901409
Yin J, LaPlant B, Uy GL et al (2019) Evaluation of event-free survival as a robust end point in untreated acute myeloid leukemia (Alliance A151614). Blood Adv 3:1714–1721. https://doi.org/10.1182/bloodadvances.2018026112
DiNardo CD, Jonas BA, Pullarkat V et al (2020) Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383:617–629. https://doi.org/10.1056/NEJMoa2012971
Johnson IM, Bezerra ED, Farrukh F et al (2022) Cardiac events in patients with acute myeloid leukemia treated with venetoclax combined with hypomethylating agents. Blood Adv 6:5227–5231. https://doi.org/10.1182/bloodadvances.2022007333
Pollyea DA, Pratz K, Letai A et al (2021) Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol 96:208–217. https://doi.org/10.1002/ajh.26039
Sandhu KS, Dadwal S, Yang D et al (2020) Outcome of allogeneic hematopoietic cell transplantation after venetoclax and hypomethylating agent therapy for acute myelogenous leukemia. Biol Blood Marrow Transplant 26:e322–e327. https://doi.org/10.1016/j.bbmt.2020.08.027
Kennedy VE, Hui G, Gaut D et al (2020) Hypomethylating agents in combination with venetoclax as a bridge to allogeneic transplant in acute myeloid leukemia. Blood 136:32–33. https://doi.org/10.1182/blood-2020-143002
Arslan S, Zhang J, Dhakal P et al (2020) Outcomes of therapy with venetoclax combined with hypomethylating agents in favorable-risk acute myeloid leukemia (AML). Blood 136:41–42. https://doi.org/10.1182/blood-2020-142780
Funding
Open Access funding enabled and organized by Projekt DEAL. David Kuron is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – project number 413490537 within the Kiel Clinician Scientist Program in Evolutionary Medicine (CSEM).
Author information
Authors and Affiliations
Contributions
D. Kuron designed and performed research, analysed data, and wrote the manuscript. A. Pohlmann, R.M. Mesters, M. Stelljes, W.E. Berdel, and G. Lenz analysed data. C. Schliemann and J.-H. Mikesch designed research, analysed data, and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective analysis involving patient data was in accordance with the ethical standards of the institutional and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. It was conducted retrospectively from data obtained for clinical purposes. It was approved by the joint ethics committee of the Physicians` Chamber Westphalia-Lippe and the University of Münster, Germany (AZ 2021–490-f-S).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christoph Schliemann and Jan-Henrik Mikesch share last authorship.
Key points
• Amsacrine-based induction therapy was associated with high early mortality in AML patients with cardiac comorbidities.
• Amsacrine-based induction cannot be recommended as alternative to standard induction therapy for AML patients with significant cardiac comorbidities in the era of novel therapies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuron, D., Pohlmann, A., Angenendt, L. et al. Amsacrine-based induction therapy in AML patients with cardiac comorbidities: a retrospective single-center analysis. Ann Hematol 102, 755–760 (2023). https://doi.org/10.1007/s00277-023-05111-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05111-x