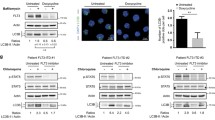
Abstract
Acute myeloid leukemia (AML) is a group of hematological malignancies characterized by clonal proliferation of immature myeloid cells. Lipid rafts are highly organized membrane subdomains enriched in cholesterol, sphingolipids, and gangliosides and play roles in regulating apoptosis through subcellular redistribution. Flotillin1 (FLOT1) is a component and also a marker of lipid rafts and had been reported to be involved in the progression of cancers and played important roles in cell death. However, the role of FLOT1 in AML remains to be explored. In this study, we found that increased expression of FLOT1 was correlated with poor clinical outcome in AML patients. Knockdown of FLOT1 in AML cells not only promoted cell death in vitro but also inhibited malignant cells engraftment in vivo. Mechanically, FLOT1 knockdown triggered apoptosis and pyroptosis. FLOT1 overexpression promoted AML cell growth and apoptosis resistance. Our findings indicate that FLOT1 is a prognostic factor of AML and may be a potential target for AML treatment.





Similar content being viewed by others
Data availability
The dataset supporting the conclusions of this article is available in the NCBI’s SRA repository. Accession to cite for the SRA data: PRJNA816556.
Abbreviations
- AML:
-
Acute myeloid leukemia
- FLOT1:
-
Flotillin1
- HHT:
-
Homoharringtonine
- ADM:
-
Doxorubicin
- Ara-C:
-
Cytarabine
- Nec-1:
-
Necrostatin-1
- Fer-1:
-
Ferrostatin-1
- Z-VAD:
-
Z-VAD(OH)-FMK
- VX-765:
-
Belnacasan
References
Döhner H, Weisdorf DJ, Bloomfield CD (2015) Acute myeloid leukemia. N Engl J Med 373(12):1136–1152
Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM (2019) Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev 36:70–87
Hanahan D (2022) Hallmarks of cancer: new dimensions. Cancer Discov 12(1):31–46
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J et al (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19(2):202–208
DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M et al (2018) Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol 19(2):216–228
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS et al (2019) Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133(1):7–17
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592
Mollinedo F, Gajate C (2020) Lipid rafts as signaling hubs in cancer cell survival/death and invasion: implications in tumor progression and therapy: thematic review series: Biology of Lipid Rafts. J Lipid Res 61(5):611–635
Garcia A, Cayla X, Fleischer A, Guergnon J, Alvarez-Franco Cañas F, Rebollo MP et al (2003) Rafts: a simple way to control apoptosis by subcellular redistribution. Biochimie 85(8):727–731
Gauthier-Rouvière C, Bodin S, Comunale F, Planchon D (2020) Flotillin membrane domains in cancer. Cancer Metastasis Rev 39(2):361–374
Kumar R, Pereira RS, Niemann J, Azimpour AI, Zanetti C, Karantanou C et al (2022) The differential role of the lipid raft-associated protein flotillin 2 for progression of myeloid leukemia. Blood Adv 6(12):3611–3624
Zhao L, Li J, Liu Y, Zhou W, Shan Y, Fan X et al (2018) Flotillin1 promotes EMT of human small cell lung cancer via TGF-β signaling pathway. Cancer Biol Med 15(4):400–414
Lin C, Wu Z, Lin X, Yu C, Shi T, Zeng Y et al (2011) Knockdown of FLOT1 impairs cell proliferation and tumorigenicity in breast cancer through upregulation of FOXO3a. Clin Cancer Res 17(10):3089–3099
Jang D, Kwon H, Choi M, Lee J, Pak Y (2019) Sumoylation of flotillin-1 promotes EMT in metastatic prostate cancer by suppressing Snail degradation. Oncogene 38(17):3248–3260
Song L, Gong H, Lin C, Wang C, Liu L, Wu J et al (2012) Flotillin-1 promotes tumor necrosis factor-α receptor signaling and activation of NF-κB in esophageal squamous cell carcinoma cells. Gastroenterology 143(4):995-1005.e12
Garbis SD, Tyritzis SI, Roumeliotis T, Zerefos P, Giannopoulou EG, Vlahou A et al (2008) Search for potential markers for prostate cancer diagnosis, prognosis and treatment in clinical tissue specimens using amine-specific isobaric tagging (iTRAQ) with two-dimensional liquid chromatography and tandem mass spectrometry. J Proteome Res 7(8):3146–3158
Fan W, Guo J, Gao B, Zhang W, Ling L, Xu T et al (2019) Flotillin-mediated endocytosis and ALIX-syntenin-1-mediated exocytosis protect the cell membrane from damage caused by necroptosis. Sci Signal 12(583)
Bagger FO, Kinalis S, Rapin N (2019) BloodSpot: a database of healthy and malignant haematopoiesis updated with purified and single cell mRNA sequencing profiles. Nucleic Acids Res 47(D1):D881–D885
Zhang Y, Chen X, Gueydan C, Han J (2018) Plasma membrane changes during programmed cell deaths. Cell Res 28(1):9–21
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H et al (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575):660–665
Ou YX, Liu FT, Chen FY, Zhu ZM (2017) Prognostic value of Flotillin-1 expression in patients with solid tumors. Oncotarget 8(32):52665–52677
Ludwig A, Otto GP, Riento K, Hams E, Fallon PG, Nichols BJ (2010) Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol 191(4):771–781
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3):486–541
Yeaton A, Cayanan G, Loghavi S, Dolgalev I, Leddin EM, Loo CE et al (2022) The impact of inflammation-induced tumor plasticity during myeloid transformation. Cancer Discov 12(10):2392–2413
Solis GP, Hoegg M, Munderloh C, Schrock Y, Malaga-Trillo E, Rivera-Milla E et al (2007) Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J 403(2):313–322
Johnson DC, Taabazuing CY, Okondo MC, Chui AJ, Rao SD, Brown FC et al (2018) DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat Med 24(8):1151–1156
Yang W, Liu S, Li Y, Wang Y, Deng Y, Sun W et al (2020) Pyridoxine induces monocyte-macrophages death as specific treatment of acute myeloid leukemia. Cancer Lett 492:96–105
Banning A, Kurrle N, Meister M, Tikkanen R (2014) Flotillins in receptor tyrosine kinase signaling and cancer. Cells 3(1):129–149
Baumann CA, Ribon V, Kanzaki M, Thurmond DC, Mora S, Shigematsu S et al (2000) CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407(6801):202–207
Amaddii M, Meister M, Banning A, Tomasovic A, Mooz J, Rajalingam K et al (2012) Flotillin-1/reggie-2 protein plays dual role in activation of receptor-tyrosine kinase/mitogen-activated protein kinase signaling. J Biol Chem 287(10):7265–7278
Kato N, Nakanishi M, Hirashima N (2006) Flotillin-1 regulates IgE receptor-mediated signaling in rat basophilic leukemia (RBL-2H3) cells. J Immunol (Baltimore, Md: 1950) 177(1):147–54
Funding
This work is supported by Key International Cooperation Projects of the National Natural Science Foundation of China (81820108004), Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022034C) and the National Natural Science Foundation of China (82170144).
Author information
Authors and Affiliations
Contributions
Sh M and YQ performed the research. JJ and JS designed the research study. XH, Js H, and Mj L contributed essential reagents and tools. Ww W, Xj L QL, Wl Y, Fl L, Jj P, Yt Z, Yc Z, and CH collected clinical samples and analyzed the data. Sh M and JS wrote the paper.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Human data have been performed in accordance with the Declaration of Helsinki and have been approved by Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (IIT20220548A). All animal experiments were reviewed and approved by the Laboratory Animal Management and Ethics Committee of Zhejiang Chinese Medical University (ZCMU) (Approval No: IACUC-20220214–11). And all animal experiments were performed following animal use guidelines and ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

SFig. 1
A. qPCR and Western blotting analysis of FLOT1 expression in human AML cell lines (n=3). B. qPCR analysis of the FLOT1 mRNA level in OCI-AML2, MV-4-11 and MOLM-13 cells transduced with FLOT1 shRNAs and control vectors (n=3). C Bar graph of gray intensity analysis of Fig. 2A (n=3). (PNG 242 kb)

SFig. 2
A. The percentage of cells in the G1, S, or G2 phase detected by flow cytometry in OCI-AML2, MV-4-11 and MOLM-13 cells transduced with FLOT1 shRNAs and control vectors. B. Flow cytometric analysis of annexin V-stained cells in primary AML cells transduced with 2 different FLOT1 shRNAs. For primary patients, the flow cytometric analysis was performed once due to limited samples. C. GSEA analysis results with normalized enrichment score (NES), NOM p-value and false discovery rate (FDR) q-value are shown for pathways enriched in MOLM-13 cells transduced with FLOT1 shRNA when compared with control (n=3). D. Bar graph of gray intensity analysis of Fig. 3E (n=3).E.Bar graph of gray intensity analysis of Fig. 3F (n=3). (PNG 189 kb)

SFig. 3
A.Bar graph of gray intensity analysis of Fig. 4A (n=3). B. Bar graph was gray intensity analysis of Fig. 4B(n=3). C. The extracellular acidification rate (ECAR) in OCI-AML2 and MOLM-13 cells transduced with FLOT1 shRNAs and control vectors(n=3)(up panel). Representative data for ECAR over time for 120 min via fluorescent emission(down panel). (PNG 194 kb)

SFig. 4
A. qPCR analysis of the FLOT1 mRNA level in THP-1 cells transduced with FLOT1 overexpression vector (n=3).B. Bar graph of gray intensity analysis of Fig, 5A (n=3). C. The percentage of cells in the G1, S, or G2 phase detected by flow cytometry in THP-1 cells transduced with FLOT1 overexpression vector (n=3). D. Bar graph of gray intensity analysis of Fig. 5D (n=3). E.qPCR analysis of the FLOT1 mRNA level in MOLM-13 cells transduced with FLOT1 overexpression vector and shRNAs vectors (n=3). F. Bar graph of gray intensity analysis of Fig. 5F (n=3). G. Bar graph of gray intensity analysis of Fig. 5H (n=3). H. PARP and FLOT1 protein levels were analyzed by Western blot in OCI-AML2 cell lines treated with Homoharringtonine(HHT), doxorubicin(ADM) or cytarabine(Ara-C) for 24 hrespectively (n=3). (PNG 408 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, S., Qian, Y., Wei, W. et al. FLOT1 knockdown inhibits growth of AML cells through triggering apoptosis and pyroptosis. Ann Hematol 102, 583–595 (2023). https://doi.org/10.1007/s00277-023-05103-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05103-x