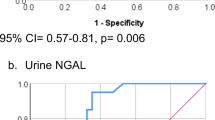
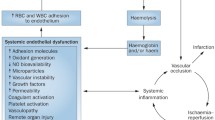
Abstract
Sickle cell disease (SCD) is a hereditary condition characterized by homozygosis of the hemoglobin S (HbS) gene. Marked morbimortality is observed due to chronic hemolysis, endothelial injury, and episodes of vaso-occlusion, which leads to multi-organ damage. Renal impairment is common and may have different presentations, such as deficiency in urinary acidification or concentration, glomerulopathies, proteinuria, and hematuria, frequently resulting in end-stage renal disease (ESRD). Novel biomarkers of renal function, such as kidney injury molecule 1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL) and monocyte chemoattractant protein 1 (MCP-1) are being studied in order to enable early diagnosis of kidney damage in SCD.




Similar content being viewed by others
References
Reddy KS (2016) Global Burden of Disease Study 2015 provides GPS for global health 2030. Lancet 388(10053):1448–1449
Sundaram N, Bennett M, Wilhelm J, Kim MO, Atweh G, Devarajan P, Malik P (2011) Biomarkers for early detection of sickle nephropathy. Am J Hematol 86(7):559–566
Hamideh D, Raj V, Harrington T, Li H, Margolles E, Amole F, Garcia-Buitrago M, Ruiz P, Zilleruelo G, Alvarez O (2014) Albuminuria correlates with hemolysis and NAG and KIM-1 in patients with sickle cell anemia. Pediatr Nephrol 29(10):1997–2003
Peres LA, Cunha Júnior AD, Schäfer AJ, Silva AL, Gaspar AD, Scarpari DF, Alves JB, Girelli Neto R, Oliveira TF (2013) Biomarkers of acute kidney injury. J Bras Nefrol 35(3):229–236
Vaidya VS, Ferguson MA, Bonventre JV (2008) Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 48:463–493
Santos TE, Gonçalves RP, Barbosa MC et al (2015) Monocyte chemoatractant protein-1: a potential biomarker of renal lesion and its relation with oxidative status in sickle cell disease. Blood Cells Mol Dis 54(3):297–301
Sanjeevani S, Pruthi S, Kalra S, Goel A, Kalra OP (2014) Role of neutrophil gelatinase-associated lipocalin for early detection of acute kidney injury. Int J Crit Illn Inj Sci 4(3):223–228
Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P (2003) Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14(10):2534–2543
Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, de Cal M, Corradi V, Virzi G, Ronco C (2010) NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol 42(1):141–150
Devarajan P (2010) Review: Neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 15(4):419–428
Serjeant GR (2010) One hundred years of sickle cell disease. Br J Haematol 151(5):425–429
Sundd P, Gladwin MT, Novelli EM (2019) Pathophysiology of sickle cell disease. Annu Rev Pathol 14:263–292
Zago MA, Pinto ACS (2007) Fisiopatologia das doenças falciformes: da mutação genética à insuficiência de múltiplos órgãos. Rev. Bras Hematol. Hemoter. Set 29(3):207–214
Adekile AD (2013) What’s new in the pathophysiology of sickle cell disease? Med Princ Pract 22(4):311–312
Kato GJ, Gladwin MT, Steinberg MH (2007) Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 21(1):37–47
Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT (2009) Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol 84(9):618–625
Rees DC, Gibson JS (2012) Biomarkers in sickle cell disease. Br J Haematol 156(4):433–445
Damanhouri GA, Jarullah J, Marouf S, Hindawi SI, Mushtaq G, Kamal MA (2015) Clinical biomarkers in sickle cell disease. Saudi J Biol Sci 22(1):24–31
Moreira JA, Laurentino MR, Machado RP, Barbosa MC, Gonçalves RP, Mota Ade M, Rocha LB, Martins AM, de Lima Arruda AB, de Souza IP, Gonçalves RP (2015) Pattern of hemolysis parameters and association with fetal hemoglobin in sickle cell anemia patients in steady state. Rev Bras Hematol Hemoter 37(3):167–171
Nath KA, Katusic ZS, Gladwin MT (2004) The perfusion paradox and vascular instability in sickle cell disease. Microcirculation 11(2):179–193
Lamarre Y, Hardy-Dessources MD, Romana M, Lalanne-Mistrih ML, Waltz X, Petras M, Doumdo L, Blanchet-Deverly A, Martino J, Tressières B, Maillard F, Tarer V, Etienne-Julan M, Connes P (2014) Relationships between systemic vascular resistance, blood rheology and nitric oxide in children with sickle cell anemia or sickle cell-hemoglobin C disease. Clin Hemorheol Microcirc 58(2):307–316
Steinberg MH (2009) Genetic etiologies for phenotypic diversity in sickle cell anemia. Sci World J 9:46–67
Orkin SH, Higgs DR (2010) Sickle cell disease at 100 years. Science. 329(5989):291–292
Maigne G, Ferlicot S, Galacteros F, Belenfant X, Ulinski T, Niaudet P, Ronco P, Godeau B, Durrbach A, Sahali S, Lang P, Lambotte O, Audard V (2010) Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 89(1):18–27
Kormann R, Jannot AS, Narjoz C, Ribeil JA, Manceau S, Delville M, Joste V, Prié D, Pouchot J, Thervet E, Courbebaisse M, Arlet JB (2017) Roles of APOL1 G1 and G2 variants in sickle cell disease patients: kidney is the main target. Br J Haematol 179(2):323–335
Bundy JL, Anderson BR, Francescatto L, Garrett ME, Soldano KL, Telen MJ, Davis EE, Ashley-Koch AE (2019) RNA sequencing of isolated cell populations expressing human APOL1 G2 risk variant reveals molecular correlates of sickle cell nephropathy in zebrafish podocytes. PLoS One 14(6):e0217042
Haymann JP, Stankovic K, Levy P, Avellino V, Tharaux PL, Letavernier E, Grateau G, Baud L, Girot R, Lionnet F (2010) Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 5(5):756–761
Becker AM (2011) Sickle cell nephropathy: challenging the conventional wisdom. Pediatr Nephrol 26(12):2099–2109
Da Silva GB, Libório AB, Daher EF (2011) New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 90(12):1371–1379
Nath KA, Hebbel RP (2015) Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol 11(3):161–171
Aban I, Baddam S, Hilliard LM, Howard TH, Feig DI, Lebensburger JD (2017) Severe anemia early in life as a risk factor for sickle-cell kidney disease. Blood. 129(3):385–387
Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y (2009) Complications associated with sickle cell trait: a brief narrative review. Am J Med 122(6):507–512
Miller ST, Wang WC, Iyer R, Rana S, Lane P, Ware RE, Li D, Rees RC, BABY-HUG Investigators (2010) Urine concentrating ability in infants with sickle cell disease: baseline data from the phase III trial of hydroxyurea (BABY HUG). Pediatr Blood Cancer 54(2):265–268
Hariri E, Mansour A, El Alam A et al (2018) Sickle cell nephropathy: an update on pathophysiology, diagnosis, and treatment. Int Urol Nephrol 50(6):1075–1083
Le Joncour A, Mesnard L, Hertig A, Robert T et al (2018) Red urine, updated for the nephrologist: a case report. BMC Nephrol 19(1):133
Pegelow CH, Colangelo L, Steinberg M, Wright EC, Smith J, Phillips G, Vichinsky E (1997) Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med 102(2):171–177
Nath KA, Katusic ZS, Gladwin MT (2004) The perfusion paradox and vascular instability in sickle cell disease. Microcirculation. 11(2):179–193
Gladwin MT (2016) Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 387(10037):2565–2574
Novelli EM, Hildesheim M, Rosano C, Vanderpool R, Simon M, Kato GJ, Gladwin MT (2014) Elevated pulse pressure is associated with hemolysis, proteinuria and chronic kidney disease in sickle cell disease. PLoS One 9(12):e114309
Gordeuk VR, Sachdev V, Taylor JG, Gladwin MT, Kato G, Castro OL (2008) Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol 83(1):15–18
Benneh-Akwasi Kuma A, Owusu-Ansah AT, Ampomah MA, Sey F, Olayemi E, Nouraie M, Ofori-Acquah SF (2018) Prevalence of relative systemic hypertension in adults with sickle cell disease in Ghana. PLoS One 13(1):e0190347
Lebensburger JD, Aban I, Pernell B, Kasztan M, Feig DI, Hilliard LM, Askenazi DJ (2019) Hyperfiltration during early childhood precedes albuminuria in pediatric sickle cell nephropathy. Am J Hematol 94(4):417–423
Bartolucci P, Habibi A, Stehlé T, di Liberto G, Rakotoson MG, Gellen-Dautremer J, Loric S, Moutereau S, Sahali D, Wagner-Ballon O, Remy P, Lang P, Grimbert P, Audureau E, Godeau B, Galacteros F, Audard V (2016) Six months of hydroxyurea reduces albuminuria in patients with sickle cell disease. J Am Soc Nephrol 27(6):1847–1853
Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C (1991) Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 115(8):614–620
Sl Y, Paul Y, Oneal P, Nouraie M (2016) Renal failure in sickle cell disease: prevalence, predictors of disease, mortality and effect on length of hospital stay. Hemoglobin 40(5):295–299
Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O'Connor MF, Devarajan P, Bonventre JV, Murray PT (2010) Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol 5(12):2154–2165 Epub 2010 Aug 26
Iannone R, Ohene-Frempong K, Fuchs EJ, Casella JF, Chen AR (2005) Bone marrow transplantation for sickle cell anemia: progress and prospects. Pediatr Blood Cancer 44(5):436–440
Kohne E (2011) Hemoglobinopathies: clinical manifestations, diagnosis, and treatment. Dtsch Arztebl Int 108(31-32):532–540
Orkin SH, Bauer DE (2019) Emerging genetic therapy for sickle cell disease. Annu Rev Med 70:257–271
Nahavandi M, Tavakkoli F, Wyche MQ, Perlin E, Winter WP, Castro O (2002) Nitric oxide and cyclic GMP levels in sickle cell patients receiving hydroxyurea. Br J Haematol 119(3):855–857
Agrawal RK, Patel RK, Shah V, Nainiwal L, Trivedi B (2014) Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfus 30(2):91–96
Nader E, Grau M, Fort R et al (2018) Hydroxyurea therapy modulates sickle cell anemia red blood cell physiology: impact on RBC deformability, oxidative stress, nitrite levels and nitric oxide synthase signalling pathway. Nitric Oxide 81:28–35
Mckie KT, Hanevold CD, Hernandez C et al (2007) Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 29(3):140–144
Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE (2013) Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 88(2):116–119
Laurin LP, Nachman PH, Desai PC et al (2014) Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 29(6):1211–1218
Roy NB, Fortin PM, Bull KR et al (2017) Interventions for chronic kidney disease in people with sickle cell disease. Cochrane Database Syst Rev 7:CD012380
Estcourt LJ, Fortin PM, Hopewell S et al (2017) Blood transfusion for preventing primary and secondary stroke in people with sickle cell disease. Cochrane Database Syst Rev 1:CD003146
Yawn BP, Buchanan GR, Afenyi-Annan AN et al (2014) Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 312(10):1033–1048
Detterich JA (2018) Simple chronic transfusion therapy, a crucial therapeutic option for sickle cell disease, improves but does not normalize blood rheology: what should be our goals for transfusion therapy? Clin Hemorheol Microcirc 68(2-3):173–186
Ware RE, de Montalembert M, Tshilolo L, Abboud MR (2017) Sickle cell disease. Lancet 390(10091):311–323
Wood JC, Cohen AR, Pressel SL, Aygun B, Imran H, Luchtman-Jones L, Thompson AA, Fuh B, Schultz WH, Davis BR, Ware RE, TWiTCH Investigators (2016) Organ iron accumulation in chronically transfused children with sickle cell anaemia: baseline results from the TWiTCH trial. Br J Haematol 172(1):122–130
Gburek J, Verroust PJ, Willnow TE, Fyfe JC, Nowacki W, Jacobsen C, Moestrup SK, Christensen EI (2002) Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J Am Soc Nephrol 13(2):423–430
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laurentino, M.R., Parente Filho, S.L.A., Parente, L.L.C. et al. Non-invasive urinary biomarkers of renal function in sickle cell disease: an overview. Ann Hematol 98, 2653–2660 (2019). https://doi.org/10.1007/s00277-019-03813-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03813-9