Abstract
Incidence and outcome of microbiologically documented bacterial/viral infections and invasive fungal disease (IFD) in children and adults after hematopoietic cell transplantation (HCT) were compared in 650 children and 3200 adults in multicenter cross-sectional nationwide study. Infections were diagnosed in 60.8% children and 35.0% adults, including respectively 69.1% and 63.5% allo-HCT, and 33.1% and 20.8% auto-HCT patients. The incidence of bacterial infections was higher in children (36.0% vs 27.6%; p < 0.0001). Infections with Gram-negative bacteria were more frequent than Gram-positives in adults (64.6% vs 44.8%; p < 0.0001). Outcome of bacterial infections was better in children (95.5% vs 91.4%; p = 0.0011). The IFD incidence (25.3% vs 6.3%; p < 0.0001) and outcome (88.0% vs 74.9%; p < 0.0001) were higher in children. The incidence of viral infections was higher in children after allo-HCT (56.3% vs 29.3%; p < 0.0001), and auto-HCT (6.6% vs 0.8%; p < 0.0001). Outcome of viral infections was better in children (98.6% vs 92.3%; p = 0.0096). Infection-related mortality was 7.8% in children and 18.4% in adults (p < 0.0001). No child after auto-HCT died of infection. Adult age, mismatched transplants, acute leukemia, chronic GVHD, CMV reactivation, infection with Gram-negatives, and duration of infection > 21 days were risk factors for death from infection. In conclusion, pediatric patients have 2.9-fold higher incidence and 2.5-fold better outcome of infections than adults after HCT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections are a significant cause of morbidity, mortality, and resource utilization after hematopoietic cell transplantation (HCT) in children and adults. Bacterial infections both after allo- and auto-HCT are known to be associated with high mortality and have become a public health problem of major concern worldwide due to antibiotic resistance. Invasive fungal disease (IFD) remains an important cause of morbidity and mortality after allo-HCT. The incidence of IFD has been reported at 9% after allo-HCT with mortality up to 50% of patients, especially after alternative donor transplantations [1,2,3]. Most studies have reported a high rate of viral infection after allo-HCT but not auto-HCT. High viral infection risk after allo-HCT is likely related to the delayed immune reconstitution after transplantation [4]. Recent EBMT (European Society for Blood and Marrow Transplantation) analysis showed that infections are responsible for 21.6% of deaths after allo-HCT and 11.0% after auto-HCT in all age groups together; however, the risk, types, and outcome of infections varied between age groups [5].
Infections occur in up to 82% of children [6,7,8] and adults [9,10,11] after HCT; however, large multicenter studies on incidence and outcome of bacterial, fungal, and viral infections are lacking. So far also no direct simultaneous comparison was made between children and adults.
In this study, we compared the incidence, type, and outcome of infections in pediatric and adult HCT centers in Poland in multicenter cross-sectional nationwide study. We analyzed also risk factors for the incidence and outcome of infections in 650 children and 3200 adults who received HCT.
Patients and methods
Design of the study
All consecutive patients transplanted between 1.01.2012 and 31.12.2015 in 5/5 pediatric, and in 11/13, adult HCT Polish centers were included in the retrospective study. Bacterial, fungal, and viral infections were reported biannually by each center and data were analyzed centrally.
Bacterial infections
Among bacterial infections, only microbiologically documented (MDI) episodes were considered. Colonizations were not included into this analysis. MDI were diagnosed as bloodstream, gut, urinary tract, respiratory tract (broncho-alveolar lavage), and skin and soft tissue infections. Bacteria were analyzed with attention to resistance profile, such as ESBL (extended-spectrum β-lactamases: bacteria producing extended-spectrum β-lactamases), AmpC (AmpC β-lactamases: bacteria producing chromosomal cephalosporinase AmpC type), KPC (Klebsiella pneumoniae carbapenemase, Enterobacteriaceae producing carbapenemase KPC type) [12], MRSA/MRSE (methicillin-resistant Staphylococcus aureus or epidermidis), or VRE (vancomycin-resistant enterococci). Multidrug resistant (MDR) bacteria denote resistance to at least two antibiotics used in empiric therapy or resistance to at least three of antibiotic classes [13, 14].
Fungal infections
The diagnosis of IFD was made according to EORTC/MSG criteria as proven, probable, or possible [15,16,17]. Patients were screened with galactomannan test mainly during neutropenia or on the basis of clinically driven indications. Diagnostics for Pneumocystis jiroveci pneumonia (PjP) was performed in case of clinical indications.
Viral infections
Viral infections were classified as episodic (diagnosed on the basis of clinical picture, and supplemented with appropriate tests) or latent (diagnosed at molecular level). The following viruses were detected by PCR analysis: adenovirus (ADV), polyoma BKV, cytomegalovirus (CMV), Epstein-Barr virus (EBV), human herpesvirus 6 (HHV-6), and community-acquired respiratory viruses (CARV) including influenza.
Supportive therapy
Uniform, standard anti-infective prophylaxis has been applied for patients undergoing HCT. Prophylactic, empirical, preemptive, or targeted anti-infectious therapy was performed with various antibacterial, antiviral, and antifungal agents according to commonly accepted strategies [13, 14, 18,19,20,21].
Prophylaxis of infections
Environmental prophylaxis was applied in all centers according to commonly accepted policy [22]. In children, antibacterial prophylaxis consisted of oral penicillin or second-generation cephalosporin (from day − 10, until neutrophil count > 1 × 109/L or end of immunosuppressive treatment) and oral gentamicin used from the beginning of conditioning until hematological recovery. Children received antifungal prophylaxis with fluconazole; from 2014, posaconazole was used in case of graft versus host disease (GVHD) or in secondary prophylaxis. In children under age of 12 years, the drug was used off-label [17] and administered according to body weight, as shown by Welzen et al [23]. In adults during neutropenia, fluoroquinolones were used for antibacterial prophylaxis and fluconazole in antifungal prophylaxis together with regular screening of serum galactomannan and computed tomography (HRCT/CT) in case of suspected IFD. Both in children and adults, acyclovir was used in prophylaxis of HSV/VZV infection until 1 year post-transplant. Weekly screening for DNA-emia and preemptive treatment were performed for CMV and EBV reactivation. Prevention of PjP included cotrimoxazole after hematopoietic recovery until the end of immunosuppressive treatment. Commercial immunoglobulin preparations were given in case of decreased immunoglobulin concentration during the first month and then monthly until B cell function recovery. Most of children receiving myeloablative conditioning (MAC) were commenced on gut rest from the first 5 days after HCT and received total parenteral nutrition (TPN) until hematopoietic recovery.
Types of transplants
Transplants were divided as autologous and allogeneic from matched sibling donors (MSD) or unrelated donors: matched (MUD) or mismatched (MMUD). Most patients who underwent MUD/MMUD-HCT received anti-thymocyte globulin (ATG) [24].
Statistical analysis
For analysis of incidence, infectious event was defined as the diagnosis of a first specific infectious disorder. Categorical variables were compared with the chi-square test, non-categorical variables were compared with the Mann-Whitney U test. Odds ratio (OR) and confidence intervals (95%CI) were calculated for the difference in occurrence of infections in patients. Cumulative 2-year incidences of bacterial, fungal, or viral infections were calculated using competing risk analysis [25], starting from the day of transplant to the day of the first infection. Death was considered as the competing event. Outcome of infection was regarded as positive in case of survival from infection or negative in case of death from infection. Infection-related mortality (IRM) was defined as any death that occurred in the presence of infection, starting from the day of diagnosis of infection. Death from infection was diagnosed as of bacterial, fungal, or viral cause; however, in many cases of IRM, patients suffered from multiple infections, and clinically the most symptomatic infection was regarded as the primary cause of death. In case of relapse and progression of malignancy, this was regarded as the primary cause of death, regardless of concomitant infection. The Kaplan-Meier method was used to determine IRM, counting from the day of diagnosis of infection. The relationship between the binary outcome, infection incidence, or death from infection, and other variables, regarded as risk factors, were analyzed using multivariate logistic regression: hazard risk (HR) and 95%CI were calculated for each factor. All reported p values are two-sided; p < 0.05 was considered as statistically significant.
Results
Overall characteristics of infections
A total number of 395/650 (60.8%) children and 1120/3200 (35.0%) adults (OR = 2.9, 95%CI = 2.0–3.6; p < 0.0001) were diagnosed for bacterial/viral MDI or IFD, including 345/499 (69.1%) and 676/1070 (63.5%) patients, respectively, after allo-HCT, while 50/151 (33.1%) and 444/2130 (20.8%) respectively, patients after auto-HCT. Patient characteristics and number of infections are shown in Table 1.
Total number of infectious episodes was 3180, including 1399 in children (2.15 per patient) and 1781 in adults (0.56 per patient) (p < 0.0001). Also respective numbers of infections per patient were higher in children for bacterial (0.88 vs 0.37; p < 0.0001), fungal (0.38 vs 0.06; p < 0.0001), and viral (0.89 vs 0.13; p < 0.0001) episodes.
Bacterial infections
Incidence
The 2-year incidence of bacterial infections was 36.0% for children and 27.6% for adult patients (p < 0.0001), including allo-HCTs (36.9% vs 41.1%, ns), and auto-HCTs (32.9% vs 20.8%; p < 0.0001) (Fig. 1a–c). These numbers were however comparable for specific primary diseases including acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia/myelodysplastic syndrome (AML/MDS), non-Hodgkin lymphoma/Hodgkin lymphoma (NHL/HD), and severe aplastic anemia (SAA). Only 12.9% adults with multiple myeloma (MM) after auto-HCT had bacterial infections (Table 2).
Infections with Gram-negative bacteria were more frequent than Gram-positive in adults (64.6%), but not in children (44.8%). The difference was highly significant (p < 0.0001; OR = 2.3, 95%CI = 1.8–2.7). The frequency of G-negative bacteria with MDR phenotype was 67.8% (158/233) in children and 47.9% (404/843) in adults (OR = 2.3; 95%CI = 1.7–3.1, p < 0.0001). The frequency of G-positive bacteria with MDR phenotype was 43.6% (125/287) in children and 40.1% (185/461) in adults (OR = 1.1; ns). Detailed etiology of G-negative and G-positive infections is presented in Tables 3 and 4.
Timing
Median time from the day of HCT to bacterial infection was 0.20 months (range − 0.2–20.6) in children and 0.23 months (range − 0.3–36.9) in adults. Median time of therapy of bacterial infection was 14 days (range 1–196; quartiles 10–21) in children and 9 days (range 1–36; quartiles 8–14) in adults (p < 0.0001).
Risk factors
In multivariate logistic analysis (Table 5), the risk of infections was higher after allo-HCT than auto-HCT (HR = 1.8; p < 0.001). In allo-HCT patients, the risk was higher in children (HR = 2.1; p < 0.001), in acute leukemia (HR = 1.6; p < 0.001), MUD vs MSD-HCT (HR = 1.6; p < 0.001), MMUD vs MSD-HCT (HR = 2.0; p < 0.001), MAC (myeloablative conditioning) vs RIC (reduced-intensity conditioning) (HR = 1.3; p < 0.001), late (> 21 days) hematological recovery (HR = 3.3; p < 0.001), acute GVHD before infection (HR = 1.7; p < 0.001), and chronic GVHD before infection (HR = 1.4; p = 0.014). In auto-HCT patients, the risk was higher in children (HR = 1.7; p < 0.001), and in patients with late (> 21 days) hematological recovery (HR = 2.8; p < 0.001). In patients with multiple myeloma (MM), the risk was lower in comparison to all other patients (HR = 0.7; p = 0.005).
Outcome
Overall outcome of bacterial infections was positive in 95.5% of infections in children and in 91.4% in adults (OR = 3.2; 95%CI = 1.6–6.5; p = 0.0011). The outcome of infections was better in children both after allo- and auto-HCT (Fig. 2a–c).
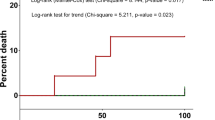
Outcome of infections. Bacterial infections a total, b allo-, and c auto-HCT. Fungal infections d total, e allo-, and f auto-HCT. With respect to level of IFD diagnosis g total, h children, and i adults. j Candida vs Aspergillus infections. Viral infections in k children vs adults. l CMV, BKV, EBV, and ADV infections. m CMV in children vs adults. n EBV in children vs adults. o ADV in children vs adults
Fungal infections
Incidence
The 2-year incidence was 25.3% for children and 6.3% for adults (p < 0.001). It was higher both in children for allo-HCTs (28.3% vs 14.0%; p < 0.0001) and auto-HCTs (14.7% vs 2.5%; p < 0.0001) (Fig. 1d–f), regardless of the level of diagnosis: proven (2.7% vs 1.6%; p < 0.0001), probable (5.7% vs 2.1%; p < 0.0001), or possible IFD (16.9% vs 2.3%; p < 0.0001). At 2 years after HCT, incidences of proven/probable IFD were 8.4% and 3.7% (p < 0.0001) for children and adults, respectively (Fig. 1g–i). The frequency was higher both in pediatric patients with ALL (29.4% vs 11.3%; OR = 3.2, p < 0.0001) and AML/MDS (41.2% vs 13.2%; OR = 4.6, p < 0.0001), when compared to adults (Table 2). Additionally, two cases of PjP infections were diagnosed (one pediatric, one adult).
Identification of fungal species
Total number of identified proven fungal infections was 74 (31 in children and 43 in adults), including 31 (42%) aspergilloses (A. spp. 6 vs 14; A. fumigatus 5 vs 2; A. flavus 2 vs 2), 34 (46%) candidiases (C. albicans 9 vs 8; C. glabrata 3 vs 4; C. krusei 1 vs 1; C. parapsilosis 1 vs 0; C. dublininsis 0 vs 1; C. guillerimondi 0 vs 1; C. kefyr 0 vs 1), 5 (6.8%) mucormycoses (Mucor spp. 1 vs 3; Rhizopus spp. 1 vs 0), and 4 (5.2%) other species (Fusarium spp. 2 vs 1; Cryptococcus spp. 0 vs 1).
Timing
Median time from day of HCT to IFD was 0.9 months (range 0–19) in children and 0.7 months (range 0–20) in adults. Median time of therapy of IFD was 24 days (range 1–590; quartiles 12–47) in children and 10 days (range 1–406; quartiles 9–26) in adults (p < 0.0001).
Risk factors
In multivariate analysis, the risk of proven/probable IFD was higher after allo-HCT than auto-HCT (HR = 5.4; p < 0.001). In allo-HCT patients, the risk was higher in children than in adults (HR = 3.9; p < 0.001), in acute leukemia (HR = 3.8; p < 0.001), MUD vs MSD-HCT (HR = 1.5; p = 0.013), MMUD vs MSD-HCT (HR = 2.5; p < 0.001), late (> 21 days) hematological recovery (HR = 3.3; p < 0.001), acute GVHD before infection (HR = 1.5; p = 0.021), and chronic GVHD before infection (HR = 2.2; p < 0.001). In auto-HCT patients, the risk was higher in children than in adults (HR = 1.8; p = 0.025). Patients with MM were at lower risk of IFD in comparison to all other patients (HR = 0.6; p = 0.005) (Table 5).
Outcome
Overall outcome of IFD was positive in 88.0% of infections in children and in 74.9% in adults (OR = 2.1; 95%CI = 1.4–3.1; p < 0.0001). The outcome of IFD was better in children than adults both after allo- and auto-HCT, regardless of the level of IFD diagnosis: proven (88.6% vs 79.6%), probable (80.8% vs 66.2%), or possible (91.4% vs 78.6%) (Fig. 2d–j).
Viral infections
Incidence
The 2-year incidence of viral infections was 56.3% for children and 29.3% for adults (p < 0.0001) after allo-HCT, and 6.6% vs 0.8% (p < 0.0001) after auto-HCT. The frequency was higher for CMV (28.9% vs 24.7%; OR = 1.7; p < 0.05), BKV (21.0% vs 5.9%; OR = 5.2; p < 0.0001), EBV (19.4% vs 1.9%; OR = 15.7; p < 0.0001), ADV (7.4% vs 2.9%; OR = 3.5; p < 0.0001), and influenza (2.2% vs 0.5%; OR = 4.8; p = 0.0038) (Table 2, Fig. 1j–l).
Viral infections after auto-HCT in children developed in 10 patients including 2 (1.3%) CMV, 1 (0.6%) influenza, 1 (0.6%) BKV, 1 (0.6%) ADV, and 5 (3.3%) rotavirus. Viral infections after auto-HCT in adults developed in 15 patients including 4 (0.18%) CMV, 5 (0.23%) influenza, 3 (0.14%) VZV, 2 (0.09%) HHV6, and 1 (0.04%) ADV.
Timing
Median time from day of HCT to viral infection was 1.4 months (range 0–19) in children and 1.6 months (range 0–21) in adults. Median time of therapy of viral infection was 13 days (range 0–168; quartiles 6–24) in children and 12 days (range 0–401; quartiles 1–28) in adults.
Risk factors
In multivariate analysis, the risk of infections was higher after allo-HCT (HR = 6.1; p < 0.001). In allo-HCT patients, the risk was higher in children (HR = 1.3; p = 0.010), in acute leukemia (HR = 1.7; p < 0.001), MUD vs MSD-HCT (HR = 2.0; p < 0.001), MMUD vs MSD-HCT (HR = 3.3; p < 0.001), MAC vs RIC (HR = 1.8; p = 0.050), acute GVHD before infection (HR = 1.5; p < 0.001), and chronic GVHD before infection (HR = 2.7; p = 0.014). Among auto-HCT patients, diagnosis of MM brought the lower risk of viral infections (HR = 0.5; p < 0.001) (Table 5).
Outcome
Overall outcome of viral infections was positive in 98.6% of infections in children and in 92.3% in adults (OR = 3.3; 95%CI = 1.2–8.7; p = 0.0096). The outcome of viral infections varied between children vs adults: CMV (97.4% vs 94.1%; p = 0.1), BKV (99.0% vs 93.9%; p = 0.075), EBV (100% vs 81.3%; p < 0.001), ADV (100% vs 96.3%; p = 0.3), and influenza (100% vs 70%; p = 0.5) (Fig. 1k–o).
Deaths from infections
Frequencies
Overall, 237 patients died from infection, including 7.8% (31/395) children and 18.4% (206/1120) adults (OR = 0.4, 95%CI = 0.3–0.6; p < 0.0001). The distribution of deaths was different in children (35.5% bacterial, 48.4% fungal, 16.1% viral) than in adults (61.7% bacterial, 24.7% fungal, 13.6% viral).
Risk factors for death from infectious complications
In allo-HCT patients, in multivariate analysis, adults (HR = 3.3; p < 0.001), recipients of MMUD-HCT (HR = 3.8; p < 0.001), patients with acute leukemia (HR = 1.5; p = 0.023), chronic GVHD before infection (HR = 3.6; p = 0.014), CMV reactivation (HR = 1.4; p = 0.038), and in patients with duration of infection treatment > 21 days (HR = 1.4; p = 0.038) were associated with increased risk of IRM (Table 6). In separate analysis of patients with bacterial infections, the risk was higher in Gram-negative in comparison to Gram-positive infections (HR = 1.6; 95%CI = 1.1–2.1; p = 0.031).
Among auto-HCT patients, no child died of infection. Among adults, the risk of death was higher if duration of treatment of infection was > 21 days (HR = 1.7; p < 0.001) (Table 6). In patients with MM, the risk was lower (HR = 0.4; p < 0.001). In separate analysis of patients with bacterial infections, there was a trend towards higher IRM in Gram-negative vs Gram-positive infections (HR = 1.8; 95%CI = 0.9–2.7; p = 0.086).
Discussion
In this study for the first time ever, simultaneous analysis and comparison of epidemiology and outcome of bacterial, fungal, and viral infections in a large cohorts of children and adults after HCT in a multicenter cross-sectional nationwide study were performed. Both groups largely differed in terms of distribution of primary diseases and their treatment, types of preparative regimens, and types of transplantation. Although both pediatric and adult transplant centers used generally very similar strategy of anti-infective management [18], some differences between these settings existed, as pediatric centers used many off-label compounds. In this study, we analyzed patients over a period of 4 years, when anti-infective prophylaxis and treatment did not change substantially in both pediatric and adult centers.
Bacterial infections occurred mainly during neutropenic, pre-engraftment phase. In adults, Gram-negative bacteria were more often documented, while in children Gram-positive species. The rate of MDR Gram-negative strains was higher in pediatric than in adult centers, while the rate of Gram-positive MDR was comparable in these cohorts. Our results indicate the shift of prevalence from Gram-positive to Gram-negative bacteria in a population of adult hematology patients and increasing incidence of MDR bacteria, especially Gram-negative [26, 27]. It is debatable, if use of quinolones in adults or oral gentamycin in children have possible negative impact in the selection of resistant gut microbiome [27]. We confirmed that irrespectively to age, transplant performed from alternative donor and prolonged neutropenia were independent risk factors for the development of bacterial infection [28]. The differences in bacterial epidemiology between children and adults resulted in differences in outcome of bacterial infections in these two cohorts, with a higher risk for death related to Gram-negative bacteria. High rate in bacterial infections was found for typical pediatric primary diagnoses like primary immunodeficiencies, neuroblastoma, and Ewing sarcoma; and opposite, in adulthood disease multiple myeloma, the rate was much lower and reached 12.9%.
Fungal infections were much more frequently diagnosed in children, regardless of the level of diagnosis; however, it was predominant for possible IFD. It reflects “real-life” pediatric strategy of reducing invasive diagnostics in children. It seems that lower incidence of IFD in adults might result from general strategy of protective environment in transplant setting and the prophylactic use of posaconazole during intensive chemotherapy in AML/MDS, according to ECIL recommendations [29,30,31]. Additionally, modified transplant procedure for pretransplant IFD, such as no-TBI conditioning, RIC, or use of PB as a stem cell source, could have possibly decrease the rate of fungal reactivations [1, 32]. Proven IFD were more often diagnosed in children. This was due mainly because of diagnosis of candidemia, as children usually have permanent, while adults rather temporarily central venous catheters. Most of children were also receiving TPN, while it was rather infrequent practice in adults. Relatively high rate of candidemia among proven IFD in children probably contributed to lower IRM than in case of invasive aspergillosis, as reported recently [33].
The incidence of viral infections was higher in children than in adults. This observation can be explained by immature immune system in children, resulting in primary infection or higher rate of reactivation of latent viruses. CMV and EBV were two most often diagnosed viruses in children after allo-HCT. CMV exerts direct and indirect effects in tissues and often plays a role of driver of another infections, including IFD, thus contributing to an increased post-transplant risk of life-threatening complications. With respect to respiratory viral infections, there is no current strategy of routine monitoring of community-acquired respiratory viruses; thus, no firm conclusion can be drawn on this topic from our study.
IRM was higher in adults, what has been evidence-proved for the first time. Additionally, IRM was higher in Gram-negative infections and in patients with acute leukemia. The outcome of infections was better in children both after allo- and auto-HCT. In addition to well-defined factors for mortality (acute leukemia, MMUD, GVHD, CMV reactivation), duration of infection > 21 days was associated with an increased risk of death after infection.
The higher infection rate of MDI in children in comparison to adults can be explained by the following factors: (1) much higher rate of auto-HCT in adults resulting in overall lower incidence of infections in adults, especially seen in case of bacterial complications; (2) higher rate of patients with acute leukemia in pediatric cohort, with a well-known high incidence of infectious complications in acute leukemia [8, 34]; (3) much higher rate of diagnosis of possible IFD in children being the consequence of the positive results of imaging only; (4) higher incidence of viral infections in children, what can correspond to higher rate of primary infections; and finally, (5) real-life tendency of pediatricians to perform more detailed diagnostic procedures. Due to the same factors, the diagnosis of multiple myeloma was associated with a decreased risk for infection in multivariate analysis. On the other hand, the incidence of infectious complications in this group of patients was similar as presented in recent analyses [35,36,37,38].
The limitation of the study is its retrospective design; however, data were collected periodically. Also no routine screening was performed for viral infections except CMV and EBV. Thus, in most cases of viral infections, the diagnosis was bound to clinical symptoms.
In conclusion, the profile of infections and related deaths largely vary between children and adults. Our study proved age-dependent determinants of pediatric and adulthood profile of infectious complications after HCT: children have higher risk of all types of infections and a better outcome of bacterial infections, while in fungal and viral infections, the IRM was comparable between children and adults. Adult age, MMUD transplants, diagnosis of acute leukemia, chronic GVHD, CMV reactivation, and infection lasting > 21 days are relative risk factors for death from infection after HCT. The potential implication of this comprehensive analysis might be differential infection control and management strategies for children and adults.
References
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, Martin PJ, Sandmaier BM, Marr KA, Appelbaum FR, Storb R, McDonald GB (2010) Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 363(22):2091–2101. https://doi.org/10.1056/NEJMoa1004383
Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA (2007) Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis 44(4):531–540
Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K (2009) Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 48(3):265–273
Ballen K, Woo Ahn K, Chen M, Abdel-Azim H, Ahmed I, Aljurf M, Antin J, Bhatt AS, Boeckh M, Chen G, Dandoy C, George B, Laughlin MJ, Lazarus HM, MacMillan ML, Margolis DA, Marks DI, Norkin M, Rosenthal J, Saad A, Savani B, Schouten HC, Storek J, Szabolcs P, Ustun C, Verneris MR, Waller EK, Weisdorf DJ, Williams KM, Wingard JR, Wirk B, Wolfs T, Young JH, Auletta J, Komanduri KV, Lindemans C, Riches ML (2016) Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 22(9):1636–1645. https://doi.org/10.1016/j.bbmt.2016.06.012
Styczynski J, Tridello G, Koster L, Iacobelli S, van Biezen A, van der Werf S, Mikulska M, Gil L, Cordonnier C, Ljungman P, Averbuch D, Cesaro C, de la Camara R, Baldomero H, Bader P, Basak G, Bonini C, Duarte RF, Dufour C, Kuball J, Lankester A, Montoto S, Nagler A, Snowden JA, Kröger N, Mohty M, Gratwohl A (2019) Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant (in press)
Srinivasan A, Wang C, Srivastava DK, Burnette K, Shenep JL, Leung W, Hayden RT (2013) Timeline, epidemiology, and risk factors for bacterial, fungal, and viral infections in children and adolescents after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 19(1):94–101. https://doi.org/10.1016/j.bbmt.2012.08.012
Srinivasan A, McLaughlin L, Wang C, Srivastava DK, Shook DR, Leung W, Hayden RT (2014) Early infections after autologous hematopoietic stem cell transplantation in children and adolescents: the St. Jude experience. Transpl Infect Dis 16(1):90–97. https://doi.org/10.1111/tid.12165
Styczynski J, Czyzewski K, Wysocki M, Gryniewicz-Kwiatkowska O, Kolodziejczyk-Gietka A, Salamonowicz M, Hutnik L, Zajac-Spychala O, Zaucha-Prazmo A, Chelmecka-Wiktorczyk L, Siewiera K, Fraczkiewicz J, Malas Z, Tomaszewska R, Irga-Jaworska N, Plonowski M, Ociepa T, Pierlejewski F, Gamrot Z, Urbanek-Dadela A, Gozdzik J, Stolpa W, Dembowska-Baginska B, Perek D, Matysiak M, Wachowiak J, Kowalczyk J, Balwierz W, Kalwak K, Chybicka A, Badowska W, Szczepanski T, Drozynska E, Krawczuk-Rybak M, Urasinski T, Mlynarski W, Woszczyk M, Karolczyk G, Sobol-Milejska G, Gil L (2016) Increased risk of infections and infection-related mortality in children undergoing haematopoietic stem cell transplantation compared to conventional anticancer therapy: a multicentre nationwide study. Clin Microbiol infect 22(2):179 e171–179 e110. https://doi.org/10.1016/j.cmi.2015.10.017
Wang L, Wang Y, Fan X, Tang W, Hu J (2015) Prevalence of resistant Gram-negative bacilli in bloodstream infection in febrile neutropenia patients undergoing hematopoietic stem cell transplantation: a single center retrospective cohort study. Medicine (Baltimore) 94(45):e1931. https://doi.org/10.1097/MD.0000000000001931
Slade M, Goldsmith S, Romee R, DiPersio JF, Dubberke ER, Westervelt P, Uy GL, Lawrence SJ (2017) Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl Infect Dis 19(1). https://doi.org/10.1111/tid.12629
Seo S, Waghmare A, Scott EM, Xie H, Kuypers JM, Hackman RC, Campbell AP, Choi SM, Leisenring WM, Jerome KR, Englund JA, Boeckh M (2017) Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica 102(6):1120–1130. https://doi.org/10.3324/haematol.2016.153767
Bassetti M, Righi E (2013) Multidrug-resistant bacteria: what is the threat? Hematology Am Soc Hematol Educ Program 2013:428–432. https://doi.org/10.1182/asheducation-2013.1.428
Averbuch D, Cordonnier C, Livermore DM, Mikulska M, Orasch C, Viscoli C, Gyssens IC, Kern WV, Klyasova G, Marchetti O, Engelhard D, Akova M (2013) Targeted therapy against multi-resistant bacteria in leukemic and hematopoietic stem cell transplant recipients: guidelines of the 4th European Conference on Infections in Leukemia (ECIL-4, 2011). Haematologica 98(12):1836–1847. https://doi.org/10.3324/haematol.2013.091330
Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, Gyssens IC, Kern WV, Klyasova G, Marchetti O, Engelhard D, Akova M (2013) European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 98(12):1826–1835. https://doi.org/10.3324/haematol.2013.091025
Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ (2002) Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34(1):7–14
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46(12):1813–1821. https://doi.org/10.1086/588660
Groll AH, Castagnola E, Cesaro S, Dalle JH, Engelhard D, Hope W, Roilides E, Styczynski J, Warris A, Lehrnbecher T (2014) Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol 15(8):e327–e340. https://doi.org/10.1016/S1470-2045(14)70017-8
Styczynski J, Gil L (2008) Prevention of infectious complications in pediatric HSCT. Bone Marrow Transplant 42(Suppl 2):S77–S81. https://doi.org/10.1038/bmt.2008.289
Maertens J, Marchetti O, Herbrecht R, Cornely OA, Fluckiger U, Frere P, Gachot B, Heinz WJ, Lass-Florl C, Ribaud P, Thiebaut A, Cordonnier C (2011) European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3–2009 update. Bone Marrow Transplant 46(5):709–718. https://doi.org/10.1038/bmt.2010.175
Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P, Styczynski J, Ward K, European Conference on Infections in L (2008) Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant 42(4):227–240. https://doi.org/10.1038/bmt.2008.162
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, Ljungman P, Engelhard D (2009) Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 43(10):757–770. https://doi.org/10.1038/bmt.2008.386
Styczynski J, Tridello G, Donnelly JP, Iacobelli S, Hoek J, Mikulska M, Aljurf M, Gil L, Cesaro S (2018) Protective environment for hematopoietic cell transplant (HSCT) recipients: the Infectious Diseases Working Party EBMT analysis of global recommendations on health-care facilities. Bone Marrow Transplant 53(9):1131–1138. https://doi.org/10.1038/s41409-018-0141-5
Welzen ME, Bruggemann RJ, Van Den Berg JM, Voogt HW, Gilissen JH, Pajkrt D, Klein N, Burger DM, Warris A (2011) A twice daily posaconazole dosing algorithm for children with chronic granulomatous disease. Pediatr Infect Dis J 30(9):794–797. https://doi.org/10.1097/INF.0b013e3182195808
Styczynski J, Debski R, Krenska A, Czyzewski K, Bartoszewicz N, Demidowicz E, Irga-Jaworska N, Drozynska E, Plonowski M, Krawczuk-Rybak M, Ociepa T, Urasiński T, Wysocki M (2017) Role of HLA match on results of hematopoietic stem cell transplantations from unrelated donors in children with acute leukemia and bone marrow failure syndromes. Acta Haematol Pol 48:48–53. https://doi.org/10.1016/j.achaem.2017.01.002
Gooley TA, Leisenring W, Crowley J, Storer BE (1999) Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 18(6):695–706. https://doi.org/10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O
Trecarichi EM, Pagano L, Candoni A, Pastore D, Cattaneo C, Fanci R, Nosari A, Caira M, Spadea A, Busca A, Vianelli N, Tumbarello M, HeMabis Registry-Seifem Group I (2015) Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect 21(4):337–343. https://doi.org/10.1016/j.cmi.2014.11.022
Mikulska M, Viscoli C, Orasch C, Livermore DM, Averbuch D, Cordonnier C, Akova M, Fourth European Conference on Infections in Leukemia Group, a joint venture of EBMT, EORTC, ICHS, ELN, ESGICH/ESCMID (2014) Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Inf Secur 68(4):321–331. https://doi.org/10.1016/j.jinf.2013.12.006
Girmenia C, Bertaina A, Piciocchi A, Perruccio K, Algarotti A, Busca A, Cattaneo C, Raiola AM, Guidi S, Iori AP, Candoni A, Irrera G, Milone G, Marcacci G, Scime R, Musso M, Cudillo L, Sica S, Castagna L, Corradini P, Marchesi F, Pastore D, Alessandrino EP, Annaloro C, Ciceri F, Santarone S, Nassi L, Farina C, Viscoli C, Rossolini GM, Bonifazi F, Rambaldi A, Gruppo Italiano Trapianto di Midollo O, Associazione Microbiologi Clinici I (2017) Incidence, risk factors and outcome of pre-engraftment Gram-negative bacteremia after allogeneic and autologous hematopoietic stem cell transplantation: an Italian prospective multicenter survey. Clin Infect Dis 65(11):1884–1896. https://doi.org/10.1093/cid/cix690
Maertens JA, Frere P, Lass-Florl C, Heinz W, Cornely OA (2007) Primary antifungal prophylaxis in leukaemia patients. EJC Suppl 5:43–38
Maertens JA, Girmenia C, Bruggemann RJ, Duarte RF, Kibbler CC, Ljungman P, Racil Z, Ribaud P, Slavin MA, Cornely OA, Peter Donnelly J, Cordonnier C, European Conference on Infections in Leukemia, joint venture of the EBMT, EORTC, ICHS, ELN (2018) European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother 73(12):3221–3230. https://doi.org/10.1093/jac/dky286
Dragonetti G, Criscuolo M, Fianchi L, Pagano L (2017) Invasive aspergillosis in acute myeloid leukemia: are we making progress in reducing mortality? Med Mycol 55(1):82–86. https://doi.org/10.1093/mmy/myw114
Martino R, Parody R, Fukuda T, Maertens J, Theunissen K, Ho A, Mufti GJ, Kroger N, Zander AR, Heim D, Paluszewska M, Selleslag D, Steinerova K, Ljungman P, Cesaro S, Nihtinen A, Cordonnier C, Vazquez L, Lopez-Duarte M, Lopez J, Cabrera R, Rovira M, Neuburger S, Cornely O, Hunter AE, Marr KA, Dornbusch HJ, Einsele H (2006) Impact of the intensity of the pretransplantation conditioning regimen in patients with prior invasive aspergillosis undergoing allogeneic hematopoietic stem cell transplantation: a retrospective survey of the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood 108(9):2928–2936
Pagano L, Dragonetti G, Cattaneo C, Marchesi F, Veggia B, Busca A, Candoni A, Prezioso L, Criscuolo M, Cesaro S, Delia M, Fanci R, Stanzani M, Ferrari A, Martino B, Melillo L, Nadali G, Simonetti E, Ballanti S, Picardi M, Castagnola C, Decembrino N, Gazzola M, Fracchiolla NS, Mancini V, Nosari A, Principe MID, Aversa F, Tumbarello M (2017) Changes in the incidence of candidemia and related mortality in patients with hematologic malignancies in the last ten years. A SEIFEM 2015-B report. Haematologica 102(10):e407–e410. https://doi.org/10.3324/haematol.2017.172536
Pelland-Marcotte MC, Hwee J, Pole JD, Nathan PC, Sung L (2019) Incidence of infections after therapy completion in children with acute lymphoblastic leukemia or acute myeloid leukemia: a systematic review of the literature. Leuk Lymphoma 1–11. https://doi.org/10.1080/10428194.2019.1573369
Chen M, Zhao Y, Xu C, Wang X, Zhang X, Mao B (2018) Immunomodulatory drugs and the risk of serious infection in multiple myeloma: systematic review and meta-analysis of randomized and observational studies. Ann Hematol 97(6):925–944. https://doi.org/10.1007/s00277-018-3284-y
Rahman S, Rybicki L, Ky Hamilton B, Pohlman B, Jagadeesh D, Cober E, Kalaycio M, Dean R, Sobecks R, Mossad SB, Majhail NS (2019) Early infectious complications after autologous hematopoietic cell transplantation for multiple myeloma. Transpl Infect Dis e13114. https://doi.org/10.1111/tid.13114
Mohan M, Susanibar-Adaniya S, Buros A, Crescencio JCR, Burgess MJ, Lusardi K, Davies F, Morgan G, Vanrhee F, Zangari M, Schinke C, Thanendrarajan S, Kothari A (2019) Bacteremias following autologous stem cell transplantation for multiple myeloma: risk factors and outcomes. Transpl Infect Dis 21(2):e13052. https://doi.org/10.1111/tid.13052
Giannopoulos K, Jamroziak K, Usnarska-Zubkiewicz L, Dytfeld D, Jurczyszyn A, Walewski J, Lech-Maranda E, Walter-Croneck A, Pienkowska-Grela B, Wrobel T, Charlinski G, Jedrzejczak WW, Malkowski B, Druzd-Sitek A, Robak T, Manko J, Giebel S, Czepko R, Meder J, Dmoszynska A (2018) Recommendations of Polish Myeloma Group concerning diagnosis and therapy of multiple myeloma and other plasmacytic dyscrasias for 2018/2019. Acta Haematol Pol 49:157–206. https://doi.org/10.2478/ahp-2018-0024
Authorship contributions
Study design: Jan Styczynski, Lidia Gil; Data analysis and interpretation: Jan Styczynski, Krzysztof Czyzewski, Przemysław Gałązka, Lidia Gil, Sebastian Giebel; Manuscript writing: Krzysztof Czyżewski, Jan Styczyński, Lidia Gil; Provision of important clinical data: All authors; Data check-up: Jan Styczyński, Krzysztof Czyżewski, Przemysław Gałązka, Lidia Gil; Statistical analysis: Krzysztof Czyżewski, Przemyslaw Gałązka, Jan Styczyński; Administrative support: Jan Styczyński, Lidia Gil; Critical revision of the manuscript: All authors; Final approval of manuscript: All authors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Czyżewski, K., Styczyński, J., Giebel, S. et al. Age-dependent determinants of infectious complications profile in children and adults after hematopoietic cell transplantation: lesson from the nationwide study. Ann Hematol 98, 2197–2211 (2019). https://doi.org/10.1007/s00277-019-03755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03755-2