Abstract
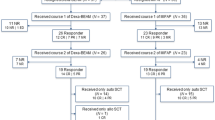
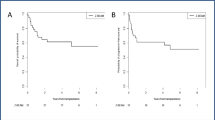
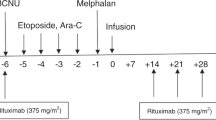
Treatment of relapse and primary progression in aggressive lymphoma remains unsatisfactory; outcome is still poor. Better treatment strategies are much needed for this patient population. The R1 study is a prospective multi-center phase I/II study evaluating a dose finding approach with a triple transplant regimen in four BEAM dose levels in patients with relapsed aggressive non-Hodgkin lymphoma. The aim of the study was to determine feasibility, toxicity, and remission rate. In a total of 39 patients (pts.) enrolled in the study, 24 pts. were evaluated in the following analysis. Twenty pts. had aggressive B cell lymphoma, and two pts. had T cell lymphoma. All evaluated patients responded to DexaBEAM with a sufficient stem cell harvest. The phase I/II study was started with BEAM dose level II. Four patients were treated at dose level II, and 20 pts. were treated at dose level III. Due to the early termination of the study, dose levels I and IV were never administered. Sixteen pts. completed therapy according to protocol, and eight pts. (33.3 %) stopped treatment early. Infections (27 %) and stomatitis (13 %) were the most frequent grade III/IV non-hematologic toxicities. Thirteen percent of patients presented with severe grade III/IV lung toxicity during modified BEAM (m-BEAM). Fourteen pts. achieved a complete response (CR), one pt. achieved no change (NC), six pts. had progressive disease (PD), and two pts. died; for one pt., outcome is not known. One-year and 3-year event-free survival (EFS) was 38 and 33 %, respectively. Overall survival (OS) after 1 and 3 years was 50 and 38 %. In conclusion, dose escalation of standard BEAM is not feasible due to toxicity.



Similar content being viewed by others
References
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL (1995) Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med 333(23):1540–1545. doi:10.1056/NEJM199512073332305
Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, Lopez-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M (2006) CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 7(5):379–391. doi:10.1016/S1470-2045(06)70664-7
Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann R, Trümper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass B, Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M (2008) Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 9:105–116
Schmitz N, Nickelsen M, Ziepert M, Haenel M, Borchmann P, Schmidt C, Viardot A, Bentz M, Peter N, Ehninger G, Doelken G, Ruebe C, Truemper L, Rosenwald A, Pfreundschuh M, Loeffler M, Glass B (2012) Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol 13(12):1250–1259. doi:10.1016/S1470-2045(12)70481-3
Gisselbrecht C, Glass B, Mounier N, Singh GD, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, Ma D, Briere J, Moskowitz CH, Schmitz N (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28(27):4184–4190. doi:10.1200/JCO.2010.28.1618
Hagberg H, Gisselbrecht C (2006) Randomised phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomisation to maintenance treatment with rituximab or not: an update of the CORAL study. Ann Oncol 17(Suppl 4):iv31–iv32. doi:10.1093/annonc/mdj996
Guglielmi C, Gomez F, Philip T, Hagenbeek A, Martelli M, Sebban C, Milpied N, Bron D, Cahn JY, Somers R, Sonneveld P, Gisselbrecht C, Van Der Lelie H, Chauvin F (1998) Time to relapse has prognostic value in patients with aggressive lymphoma enrolled onto the Parma trial. J Clin Oncol 16(10):3264–3269
Mills W, Strang J, Goldstone AH, Linch DC (1995) Dose intensification of etoposide in the BEAM ABMT protocol for malignant lymphoma. Leuk Lymphoma 17(3-4):263–270. doi:10.3109/10428199509056831
Dreger P, Marquardt P, Haferlach T, Jacobs S, Mulverstedt T, Eckstein V, Suttorp M, Loffler H, Muller-Ruchholtz W, Schmitz N (1993) Effective mobilisation of peripheral blood progenitor cells with ‘Dexa-BEAM’ and G-CSF: timing of harvesting and composition of the leukapheresis product. Br J Cancer 68(5):950–957
Pfreundschuh MG, Rueffer U, Lathan B, Schmitz N, Brosteanu O, Hasenclever D, Haas R, Kirchner H, Koch P, Kuse R et al (1994) Dexa-BEAM in patients with Hodgkin’s disease refractory to multidrug chemotherapy regimens: a trial of the German Hodgkin’s Disease Study Group. J Clin Oncol 12(3):580–586
O’Quigley J, Shen LZ (1996) Continual reassessment method: a likelihood approach. Biometrics 52(2):673–684
Garrett-Mayer E (2006) The continual reassessment method for dose-finding studies: a tutorial. Clin Trials 3(1):57–71
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, Thomas ED (1988) Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 6(10):1562–1568
Gisselbrecht C, Bosly A, Lepage E, Reyes F, Philip T, Haioun C, Tilly H, Coiffier B (1993) Autologous hematopoietic stem cell transplantation in intermediate and high grade non-Hodgkin’s lymphoma: a review. Ann Oncol 4(Suppl 1):7–13
Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, Colombat P, Goldstone AH, Gorin NC, Flesh M et al (1987) High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. N Engl J Med 316(24):1493–1498. doi:10.1056/NEJM198706113162401
Armitage JO, Vose JM, Bierman PJ, Bishop MR (1994) Salvage therapy for patients with lymphoma. Semin Oncol 21(4 Suppl 7):82–85
Kirschey S, Flohr T, Wolf HH, Frickhofen N, Gramatzki M, Link H, Basara N, Peter N, Meyer RG, Schmitz N, Weidmann E, Banat A, Schulz A, Kolbe K, Derigs G, Theobald M, Hess G (2014) Rituximab combined with DexaBEAM followed by high dose therapy as salvage therapy in patients with relapsed or refractory B-cell lymphoma: mature results of a phase II multicentre study. Br J Haematol. doi:10.1111/bjh.13234
Schmitz N, Diehl V (1997) Carmustine and the lungs. Lancet 349(9067):1712–1713. doi:10.1016/S0140-6736(05)62951-0
Wheeler C, Antin JH, Churchill WH, Come SE, Smith BR, Bubley GJ, Rosenthal DS, Rappaport JM, Ault KA, Schnipper LE et al (1990) Cyclophosphamide, carmustine, and etoposide with autologous bone marrow transplantation in refractory Hodgkin’s disease and non-Hodgkin’s lymphoma: a dose-finding study. J Clin Oncol 8(4):648–656
Zelenetz AD, Hamlin P, Kewalramani T, Yahalom J, Nimer S, Moskowitz CH (2003) Ifosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin’s lymphoma. Ann Oncol 14(Suppl 1):i5–i10
Gisselbrecht C (2012) Is there any role for transplantation in the rituximab era for diffuse large B-cell lymphoma? Hematology Am Soc Hematol Educ Program 2012:410–416. doi:10.1182/asheducation-2012.1.410
Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, Rubinger M, Buckstein R, Imrie KR, Federico M, Di Renzo N, Howson-Jan K, Baetz T, Kaizer L, Voralia M, Olney HJ, Turner AR, Sussman J, Hay AE, Djurfeldt MS, Meyer RM, Chen BE, Shepherd LE (2014) Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol 32(31):3490–3496. doi:10.1200/JCO.2013.53.9593
Monjanel H, Deconinck E, Perrodeau E, Gastinne T, Delwail V, Moreau A, Francois S, Berthou C, Gyan E, Milpied N, Goelams (2011) Long-term follow-up of tandem high-dose therapy with autologous stem cell support for adults with high-risk age-adjusted international prognostic index aggressive non-Hodgkin lymphomas: a GOELAMS pilot study. Biol Blood Marrow Transplant 17(6):935–940. doi:10.1016/j.bbmt.2010.11.017
Koenigsmann M, Casper J, Kahl C, Basara N, Sayer HG, Behre G, Theurich S, Christopeit M, Mohren M, Reichle A, Metzner B, Ganser A, Stadler M, Uharek L, Balleisen L, Hinke A, Hinke R, Niederwieser D (2014) Risk-adapted, treosulfan-based therapy with auto- and allo-SCT for relapsed/refractory aggressive NHL: a prospective phase-II trial. Bone Marrow Transplant 49(3):410–415. doi:10.1038/bmt.2013.199
Shimoni A, Avivi I, Rowe JM, Yeshurun M, Levi I, Or R, Patachenko P, Avigdor A, Zwas T, Nagler A (2012) A randomized study comparing yttrium-90 ibritumomab tiuxetan (Zevalin) and high-dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma. Cancer 118(19):4706–4714. doi:10.1002/cncr.27418
Intlekofer AM, Younes A (2014) Precision therapy for lymphoma—current state and future directions. Nat Rev Clin Oncol 11(10):585–596. doi:10.1038/nrclinonc.2014.137
Batlevi CL, Matsuki E, Brentjens RJ, Younes A (2015) Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. doi:10.1038/nrclinonc.2015.187
Maddocks K, Christian B, Jaglowski S, Flynn J, Jones JA, Porcu P, Wei L, Jenkins C, Lozanski G, Byrd JC, Blum KA (2015) A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood 125(2):242–248. doi:10.1182/blood-2014-08-597914
Glass B, Hasenkamp J, Wulf G, Dreger P, Pfreundschuh M, Gramatzki M, Silling G, Wilhelm C, Zeis M, Gorlitz A, Pfeiffer S, Hilgers R, Truemper L, Schmitz N, German High-Grade Lymphoma Study G (2014) Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol 15(7):757–766. doi:10.1016/S1470-2045(14)70161-5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Karin Hohloch and Samira Zeynalova contributed equally to this work.
Appendix
Rights and permissions
About this article
Cite this article
Hohloch, K., Zeynalova, S., Chapuy, B. et al. Modified BEAM with triple autologous stem cell transplantation for patients with relapsed aggressive non-Hodgkin lymphoma. Ann Hematol 95, 1121–1128 (2016). https://doi.org/10.1007/s00277-016-2671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2671-5