Abstract
Purpose
To investigate feasibility and outcomes of endovascular repair for acute thoracoabdominal aortic aneurysms (TAAA).
Materials and Methods
Data from a single center were retrospectively analyzed. Patients who underwent endovascular repair for acute TAAA between January 2010 and April 2020 were included. Perioperative and mid-term follow-up outcomes were analyzed. Survival, freedom from reintervention, and target vessel patency were calculated by Kaplan–Meier analysis.
Results
A total of 30 patients (18 men, 67.5 ± 6.9 years) underwent endovascular repair for acute symptomatic (n = 15) or contained ruptured (n = 15) TAAA. An off-the-shelf four-branched stent-graft (T-Branch) was used in 19 (63.3%) patients, a custom-made device (CMD) with expedite order in 5 (16.7%) patients, a CMD with short anticipated delivery time in 3 (10.0%) patients, and a CMD available in the hospital in 3 (10.0%) patients. Technical success was 90.0% (n = 27). Thirty-day mortality was 10% (n = 3). There was no complete persistent paraplegia, but one (3.3%) patient suffered permanent limb weakness. Estimated survival at 1 and 2 years was 86.3% ± 6.4%, and 82.3% ± 7.2%, respectively. Estimated freedom from reintervention at 1 and 2 years was 81.4% ± 7.6% and 73% ± 8.8%. Estimated target vessel patency at 1 and 2 years was 96.6% ± 2% and 92.6% ± 2.9%.
Conclusion
Endovascular treatment of acute TAAA in this selected group of patients was associated with low early mortality and excellent mid-term survival. The off-the-shelf stent-graft option (T-Branch) was used in the majority of patients. Endovascular repair should be considered the first option for suitable acute TAAA.
Similar content being viewed by others
Introduction
Treatment of acute symptomatic or ruptured thoracoabdominal aortic aneurysms (TAAA) represents a major challenge for vascular surgeons. Open repair is associated with significant mortality and morbidity. Besides, a substantial proportion of patients is judged unfit and turned down for open repair [1,2,3]. Endovascular repair of elective TAAA using custom-made fenestrated and branched stent-grafts is now increasingly applied, and some institutions represent the first line treatment for most patients. Results from high-volume centers show good early and mid-term outcome [4,5,6,7,8]. Endovascular repair of acute TAAA using custom-made fenestrated and branched stent-grafts is not a realistic option for the majority of the cases in view of the longer planning and manufacturing process. The first attempts of endovascular repair of acute ruptured TAAA included the use of surgeon-modified fenestrated stent-grafts, and different combinations of parallel-graft techniques (chimney, periscope, sandwich) [9, 10]. For acute non-ruptured (symptomatic) TAAA, custom-made fenestrated and branched devices with expedite order have been used. In recent years (since 2012), an off-the-shelf 4-branch stent-graft (T-Branch, Cook Medical, Bloomington, Indiana, USA) became available. This off-the-shelf graft has been used in elective cases with excellent short-term outcomes [11, 12]. A recent systematic review analyzing a total of seven studies has also demonstrated feasibility of the T-Branch in urgent cases [13]. Overall, the available literature reporting endovascular treatment of acute TAAA remains limited [14].
The aim of this study was to present a single center experience on endovascular treatment of acute TAAA, focusing on technique, graft choice, and clinical outcomes.
Materials and Methods
Data of patients who presented with an acute TAAA (acute symptomatic or contained rupture) within the period January 2010 and April 2020 were collected and reviewed. Patients that underwent endovascular repair were further analyzed. Patients that were treated by open repair or died before/without treatment (but with intention-to-treat) during the study period are also reported for completeness. The study was registered in our institutional study center.
Acute TAAA was defined as acute symptomatic based on clinical presentation in the presence of a TAAA but without evidence of rupture on computer tomography angiography (CTA) and contained ruptured with proven periaortic hematoma on a preoperative CTA. Patients with suprarenal aortic aneurysms treated with fenestrated/branched grafts, even if including all four visceral vessels, were excluded.
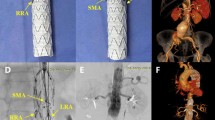
Procedure details have been previously described [5]. Stent-grafts used included the off-the-shelf 4-branch graft (T-Branch, Cook Medical, Bloomington, Indiana, USA), and custom-made devices (CMD) (available in the hospital, or with expedite order, or with short anticipated delivery time). Bridging stent-grafts used included the balloon expandable Atrium Advanta V12 (Maquet Getinge Group, Hudson, NH, USA), the Begraft Plus (Bentley InnoMed GmbH, Hechingen, Germany), and the Lifestream (Bard Peripheral Vascular, Tempe, AZ, USA). When a longer covered bridging stent was required, the Fluency (Bard Peripheral Vascular, Tempe, AZ, USA) or the Viabahn (W. L. Gore, Flagstaff, AZ, USA) was used. Technical success was defined as successful deployment of the stent-grafts by endovascular means only, absence of type I or III endoleak at the completion angiography, and patent target vessels. Postoperatively, patients received a CTA control prior to discharge and thereafter depending on each patient’s characteristics (most commonly annually).
Unless contraindicated, patients were routinely discharged on double antiplatelet treatment for at least 6 months and then monotherapy with aspirin/clopidogrel. In case that oral anticoagulation was indicated for other comorbidity (e.g., atrial fibrillation) patients were discharged on aspirin and DOAC (direct oral anticoagulant).
Data Analysis
Data were processed using the SPSS 22 (IBM Corp, Armonk, NY, USA). Collected data included patient demographics, medical comorbidity and risk-factors, TAAA diameter and type according to modified Crawford classification, stent-graft details, operative data (procedure duration, fluoroscopy duration, contrast volume, use of cerebrospinal fluid-CSF drainage), technical success, major perioperative complications, spinal cord ischemia (SCI), perioperative mortality, and late events with regard to mortality, reinterventions, and target vessel occlusion during follow-up. Survival, target vessel stent patency and reintervention during follow-up were analyzed with Kaplan–Meier. Variables are presented as mean ± standard deviation (SD) in case of normal distribution and median plus range in case of skewed distribution.
Results
During the study period, a total of 41 patients presented with acute TAAA. Six patients underwent open repair (surgical mortality: n = 5), and five patients died before/without treatment (Table 1). Thirty patients were treated by endovascular means for acute symptomatic (n = 15) or contained ruptured (n = 15) TAAA and were further analyzed in this study. Mean age was 67.5 ± 6.9 years. The demographic data of patients treated by endovascular means are summarized in Table 2.
Aneurysm and Stent-Graft Characteristics
The mean TAAA diameter was 77.8 ± 15 mm. Extent of TAAA was: I: n = 3 (10.0%), II: n = 4 (13.3%), III: n = 9 (30.0%), IV: n = 12 (40.0%), V: n = 2 (6.7%). An off-the-shelf four-branched stent-graft (T-Branch) was used in 19 (63.3%) patients, a custom-made device (CMD) with expedite order in 5 (16.7%) patients, a CMD with short anticipated delivery time (graft already ordered electively, TAAA became acute during waiting time) in 3 (10.0%) patients, and a CMD available in the hospital in 3 (10.0%) patients. The time interval between admission and treatment for the eight patients that had to wait for a CMD ranged between 4 and 22 days. A total of 112 visceral/renal vessels were targeted (mean 3.7/patient).
Operative Data
Mean operative time was 232 ± 75 min with mean fluoroscopy time of 62 ± 21 min and mean contrast volume of 192 ± 55 ml. Spinal catheter for cerebrospinal fluid (CSF) drainage was used in 15 (50.0%) patients.
Technical Success
Technical success by endovascular means only was achieved in 27 (90.0%) patients. Two patients required an additional laparotomy intraoperatively for retrograde target vessel catheterization (one patient due to false mating of the branch for the celiac artery with the superior mesenteric artery, one patient due to failed antegrade bridging of the left renal artery due to long distance and bad angle). In the third patient, the procedure was not completed as the patient died intraoperatively. This patient was initially planned to be treated electively with a 5 × fenestrated CMD (3 renal arteries, superior mesenteric artery, celiac artery) for a type IV TAAA, but suffered a rupture before the planned operation. He was admitted in an unstable condition, and it was attempted to treat him with the CMD that was already delivered. The patient died shortly after graft introduction and before catheterization of target vessels could be initiated. It is to note that his elective procedure had been delayed due to the COVID-19 pandemic.
Perioperative Mortality and Spinal Cord Ischemia
Thirty-day mortality was 10% (3 patients). One patient with rupture died intraoperatively as already described. One patient with a symptomatic TAAA underwent a technically successful procedure, but suffered a subdural hematoma 6 days postoperatively and died after a neurosurgical procedure. The third patient, with a rupture, died of multiple organ failure 5 days postoperatively.
Median duration of postoperative hospital stay was 10 (6–29) days and median duration of ICU stay was three (1–12) days. There was no complete persistent paraplegia. Four (13.3%) patients (two with symptomatic, two with contained ruptured TAAA) developed spinal cord ischemia (SCI) symptoms, of which three (10.0%) with temporary and one (3.3%) with permanent limb weakness.
Follow-up
Median follow-up was 26 months (range 1–125). Estimated survival at 1 and 2 years was 86.3% ± 6.4%, and 82.3% ± 7.2%, respectively (Fig. 1). During late follow-up, five patients died, none of them related to the TAAA. Estimated freedom from reintervention at 1 and 2 years was 81.4% ± 7.6% and 73% ± 8.8%, respectively (Fig. 2). The majority of reinterventions (66.7%) were performed by endovascular means (Table 3). Estimated target vessel patency at 1 and 2 years was 96.6% ± 2% and 92.6% ± 2.9%, respectively (Fig. 3). Overall, seven renal arteries (all targeted with downward branches) occluded in five patients treated with the off-the-shelf T-branch graft. Two of these patients suffered bilateral renal artery occlusion and required permanent dialysis despite implantation of splenorenal/hepatorenal surgical bypasses. No occlusions were noted in CMD stent-grafts.
Discussion
Endovascular treatment of elective TAAAs is being increasingly applied, and multiple centers have reported their outcomes mainly with the use of fenestrated and branched stent-grafts [4,5,6, 12, 15]. Endovascular treatment of acute TAAAs is reported only in smaller numbers in single center series. Initial reports focused mainly on chimney/sandwich techniques or surgeon-modified stent-grafts. [9, 10, 16] More recently few series have reported also the use of fenestrated and branched stent-grafts and particularly the use of the off-the-shelf T-Branch stent-graft for acute TAAA repair [17, 18].
The present series reports endovascular repair of acute TAAAs with fenestrated and branched grafts. The study showed a thirty-day mortality of 10%, which is low given the nature of the disease, and compares favorably to historical open surgical cohorts with reported perioperative mortality of around 50% [3, 19]. Many of the patients were referred from other hospitals, which demonstrates that acute TAAA can remain stable for some time.
A technical success rate of 90% shows a trend toward slightly inferior rates compared with elective series. Treatment of patients presenting acutely with symptoms or rupture is more challenging. The disease process is often more advanced in larger aneurysms (with a mean diameter of almost 8 cm) and therefore more likely to present with adverse anatomy (angulated aorta and target vessels). Additionally, most patients in the series were treated with an off-the- shelf device that did not perfectly match the anatomy of each patient. The T-Branch was used in 63% of the patients showing overall a good applicability as an off-the-shelf option, at least in experienced hands. Extra technical difficulties should be expected (larger distance of branches to target vessels, suboptimal orientation of the branches), which results in need of extra materials that should be readily available (longer bridging stents, snares, etc.). Technical difficulties can also require additional unplanned maneuvers (e.g., retrograde target vessel catheterization) to achieve technical success.
Spinal cord ischemia was noted in 13.3% of the patients, as expected in view of the higher risk of hemodynamic instability, but permanent limb weakness remained below 5%, which is a favorable outcome. Prophylactic CSF drainage was used in 50% of the patients. There was no statistically significant difference in the incidence of SCI among patients with and without prophylactic CSF drainage. A general conclusion for/against prophylactic CSF drainage in acute TAAA patients is not possible from the present retrospective analysis due to several confounding factors and biases (e.g., small patient cohort, patients chosen for CSF drainage have a higher estimated risk for SCI to start with, etc.). Extent of the repair, patency of the left subclavian and hypogastric arteries, acuity of the procedure, anticoagulation/antiplatelet medication (not stopped), and presence of an experienced specialized anesthesiologist should altogether drive an individualized decision toward insertion of a spinal catheter. Gradually, we tend to become more conservative with the prophylactic use of CSF drainage in elective cases, using it mostly for type II repairs only. For all other cases, CSF drainage is placed after the procedure in case SCI symptoms occur.
Long-term outcome of acute endovascular TAAA repair is not well documented in the literature. The estimated survival rates of > 80% at two years and the absence of aneurysm related deaths during follow-up seem to demonstrate successful exclusion of the aneurysm in most cases. Target vessel patency remained overall good (> 90% after 2 years), although as reported above two patients suffered bilateral renal artery branch occlusion requiring finally permanent dialysis. It is difficult based on such small patient cohorts to make comparisons with elective TAAA, but we may see a trend toward slightly lower target vessel patency rates for acute TAAA compared to our elective TAAA series. This could be attributed to a more adverse target vessel anatomy in larger acute TAAAs and the emergency nature of the repair. The use of an off-the-shelf branched device rather than a more matching CMD device may also play a role (e.g., longer distances to be bridged). In addition, some renal arteries might have been more suitable for a fenestration instead of a branch, but the unavailability of off-the-shelf fenestrated grafts made this selection impossible in the acute setting. This is important given previously reported data showing that branches for renal arteries may have inferior patency rates compared to fenestrations [20].
The group of Birmingham reported recently their experience with a total of 39 patients treated for an acute symptomatic or ruptured TAAA [14]. Surgeon-modified fenestrated grafts were used in the majority (61.5%) of the patients, and the T-Branch in 33% of the patients. The 30-day mortality was 26%. SCI was noted in 10%, with paraplegia in 5.1%. Estimated survival at 1 and 2 years was 71.8 ± 7.2% and 63.2 ± 7.9%, respectively. Estimated freedom from re-intervention at 1 and 2 years was 93 ± 4.8% and 85.3 ± 6.8%, respectively.
Beyond the 30 patients that were treated by endovascular means, 11 additional patients presented with an acute TAAA during the study period (Table 1). Six of those patients were treated by open means, of which five (83.3%) died, and five patients died before treatment could be initiated. Overall mortality among patients presenting with an acute TAAA was therefore 31.7% (13/41). The reason that drove the decision for open repair in six patients was anatomical in three patients (no acute endovascular option feasible), severe hemodynamic instability in one patient, and septic rupture in one patient, where we opted for a bovine pericardium graft. The sixth patient was initially planned for elective F/BEVAR, but became symptomatic during the waiting time and was admitted at his local hospital. The option to wait until the ordered graft would be available was considered, but the local surgeon decided to proceed with open repair.
Five patients died before treatment, three of those while waiting for a T-Branch to be delivered. In the past, there were time periods that a T-Branch was not always available at the hospital due to manufacturing and logistical issues. Nowadays these issues have been solved, and we aim to have three off-the-shelf branched grafts available at all times: a “standard” T-Branch, a low profile T-Branch, and a T-Branch with a proximal diameter of 38 mm instead of 34 mm. The larger proximal diameter eliminates the need for a higher proximal start with a tube graft first in patients with larger aortas, simplifying the procedure and reducing aortic coverage length. To our opinion such a modified T-Branch with a larger proximal diameter of 38 mm should become widely available. At this moment, we still order the last two options as a CMD that we keep available for acute patients.
One patient died during waiting for an expedite order of a CMD with three branches (left renal artery too small to be stented). Retrospectively, a T-Branch could have been used with adjunct occlusion of the fourth branch with an Amplatzer plug avoiding manufacturing delay for the CMD graft. After overcoming the initial learning curve period, we now favor more frequently a T-Branch graft over a CMD with expedite order opting for quicker treatment over ideal anatomy match. Indeed, the T-Branch is nowadays the most commonly used graft in acute TAAAs as an off-the-shelf option. The use of a CMD is considered only in few individual cases that the anatomy is not suitable for a T-Branch, and there is a realistic possibility to use a CMD (e.g., CMD already in hospital or with short anticipated delivery time). This strategy is followed in acute symptomatic & contained ruptured TAAAs, but also in elective large TAAAs, aiming to reduce the risk of rupture during waiting time [21].
This study has several limitations. Patients included were per definition representing a selected population as hemodynamically stable enough to overthink a treatment plan and sometimes even waiting days until a graft became available. A high proportion of patients were referred from other hospitals creating an additional selection bias (only stable patients making it to the referral center). Heterogeneity of the included patients in terms of anatomy and clinical status (symptomatic-rupture) should be acknowledged. The study reflects outcomes over a long study period, during which there have been continuous changes with regard to stent-graft technology and surgeons’ experience in complex endovascular aortic repair. Finally, the reported data reflect outcomes of a high-volume center with extensive experience in endovascular repair of elective TAAAs.
Conclusions
Endovascular repair of acute TAAA in well selected patients is associated with low mortality and excellent early and mid-term results. Endovascular treatment should be considered the first treatment option for patients with suitable acute TAAA. The T-branch appears to be an excellent off-the-shelf option for an important proportion of patients presenting with acute TAAA and should be used more liberally.
References
Coselli JS, LeMaire SA, Preventza O, de la Cruz KI, Cooley DA, Price MD, Stolz AP, Green SY, Arredondo CN, Rosengart TK. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thor Cardiovasc Surg. 2016;151(5):1323–37.
Hansen PA, Richards JM, Tambyraja AL, Khan LR, Chalmers RT. Natural history of thoraco-abdominal aneurysm in high-risk patients. Eur J Vasc Endovasc Surg. 2010;39(3):266–70.
Cowan JA Jr, Dimick JB, Wainess RM, Henke PK, Stanley JC, Upchurch GR Jr. Ruptured thoracoabdominal aortic aneurysm treatment in the United States: 1988 to 1998. J Vasc Surg. 2003;38(2):319–22.
Guillou M, Bianchini A, Sobocinski J, Maurel B, D’Elia P, Tyrrell M, Azzaoui R, Haulon S. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg. 2012;56(1):65–73.
Verhoeven EL, Katsargyris A, Bekkema F, Oikonomou K, Zeebregts CJ, Ritter W, et al. Editor’s Choice - Ten-year Experience with Endovascular Repair of Thoracoabdominal Aortic Aneurysms: Results from 166 Consecutive Patients. Eur J Vasc Endovasc Surg. 2015;49:524–31.
Rossi SH, Patel A, Saha P, Gwozdz A, Salter R, Gkoutzios P, Carrell T, Abisi S, Modarai B. Neuroprotective strategies can prevent permanent paraplegia in the majority of patients who develop spinal cord ischaemia after endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2015;50(5):599–607.
Clough RE, Modarai B, Bell RE, Salter R, Sabharwal T, Taylor PR, Carrell TW. Total endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2012;43(3):262–7.
Budtz-Lilly J, Wanhainen A, Eriksson J, Mani K. Adapting to a total endovascular approach for complex aortic aneurysm repair: outcomes after fenestrated and branched endovascular aortic repair. J Vasc Surg. 2017;66:1349–56.
Kolvenbach RR, Yoshida R, Pinter L, Zhu Y, Lin F. Urgent endovascular treatment of thoraco-abdominal aneurysms using a sandwich technique and chimney grafts–a technical description. Eur J Vasc Endovasc Surg. 2011;41(1):54–60.
Ricotta JJ 2nd, Tsilimparis N. Surgeon-modified fenestrated-branched stent grafts to treat emergently ruptured and symptomatic complex aortic aneurysms in high-risk patients. J Vasc Surg. 2012;56(6):1535–42.
Gasper WJ, Reilly LM, Rapp JH, Grenon SM, Hiramoto JS, Sobel JD, Chuter TA: Assessing the anatomic applicability of the multibranched endovascular repair of thoracoabdominal aortic aneurysm technique. J Vasc Surg 2013, 57(6):1553–1558; discussion 1558.
Bisdas T, Donas KP, Bosiers M, Torsello G, Austermann M. Anatomical suitability of the T-branch stent-graft in patients with thoracoabdominal aortic aneurysms treated using custom-made multibranched endografts. J Endovasc Ther. 2013;20(5):672–7.
Konstantinou N, Antonopoulos CN, Jerkku T, Banafsche R, Kolbel T, Fiorucci B, Tsilimparis N. Systematic review and meta-analysis of published studies on endovascular repair of thoracoabdominal aortic aneurysms with the t-Branch off-the-shelf multibranched endograft. J Vasc Surg. 2020;72(2):716-725 e711.
Mascoli C, Vezzosi M, Koutsoumpelis A, Iafrancesco M, Ranasinghe A, Clift P, Mascaro J, Claridge M, Adam DJ. Endovascular Repair of Acute Thoraco-abdominal Aortic Aneurysms. Eur J Vasc Endovasc Surg. 2018;55(1):92–100.
Tsilimparis N, Fiorucci B, Debus ES, Rohlffs F, Kolbel T. Technical aspects of implanting the t-branch off-the-shelf multibranched stent-graft for thoracoabdominal aneurysms. J Endovasc Ther. 2017;24(3):397–404.
Pecoraro F, Pfammatter T, Mayer D, Frauenfelder T, Papadimitriou D, Hechelhammer L, Veith FJ, Lachat M, Rancic Z. Multiple periscope and chimney grafts to treat ruptured thoracoabdominal and pararenal aortic aneurysms. J Endovasc Ther. 2011;18(5):642–9.
Gallitto E, Gargiulo M, Freyrie A, Pini R, Mascoli C, Ancetti S, Faggioli G, Stella A. Off-the-shelf multibranched endograft for urgent endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2017;66:696–704.
Wolosker N, Fioranelli A, Ferreira M, Tachibana A, Lembranca L, Oliveira C. Endovascular repair of ruptured thoracoabdominal aortic aneurysm with an off-the-shelf endoprosthesis. Ann Vasc Surg. 2017;43:312.
Rigberg DA, McGory ML, Zingmond DS, Maggard MA, Agustin M, Lawrence PF, Ko CY: Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg 2006, 43(2):217–222; discussion 223.
Martin-Gonzalez T, Mastracci T, Carrell T, Constantinou J, Dias N, Katsargyris A, Modarai B, Resch T, Verhoeven E, Haulon S. Mid-term outcomes of renal branches versus renal fenestrations for thoraco-abdominal aneurysm repair. Eur J Vasc Endovasc Surg. 2016;52(2):141–8.
Katsargyris A, Uthayakumar V, MarquesdeMarino P, Botos B, Verhoeven EL. Aneurysm rupture and mortality during the waiting time for a customised fenestrated/branched stent graft in complex endovascular aortic repair. Eur J Vasc Endovasc Surg. 2020;60(1):44–8.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Athanasios Katsargyris has received speaker fees from Cook Inc. and is a consultant for Bentely InnoMed. Pablo Marques de Marino, Balazs Botos, Sebastian Nagel, and Anas Ibraheem declare that have no conflict of interest. Eric LG Verhoeven has received research grants from Cook Inc. and honoraries as speaker and consultant from Cook Inc, Maquet Getinge and Bentley InnoMed..
Consent for Publication
For this type of study consent for publication is not required.
Ethical Approval
For this type of study formal consent is not required.
Informed Consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katsargyris, A., de Marino, P.M., Botos, B. et al. Single Center Experience with Endovascular Repair of Acute Thoracoabdominal Aortic Aneurysms. Cardiovasc Intervent Radiol 44, 885–891 (2021). https://doi.org/10.1007/s00270-021-02798-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-021-02798-1