Abstract
Objective
To compare temperature, energy, and coagulation between hydrochloric acid-infused radiofrequency ablation (HAIRFA) and normal saline-infused radiofrequency ablation (NSIRFA) in ex vivo porcine liver model.
Materials and Methods
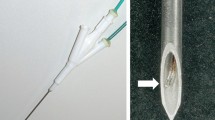
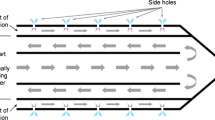
30 fresh porcine livers were excised in 60 lesions, 30 with HAIRFA and the other 30 with NSIRFA. Both modalities used monopolar perfusion electrode connected to a RF generator set at 103 °C and 30 W. In each group, ablation time was set at 10, 20, or 30 min (10 lesions from each group at each time). We compared tissue temperatures (at 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 cm away from the electrode tip), average power, deposited energy, deposited energy per coagulation volume (DEV), coagulation diameters, coagulative volume, and spherical ratio between the two groups.
Results
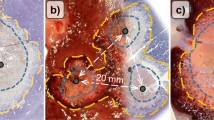
Temperature–time curves showed that HAIRFA provided progressively greater heating than that of NSIRFA. At 30 min, mean average power, deposited energy, coagulation volumes (113.67 vs. 12.28 cm3) and diameters, and increasing in tissue temperature were much greater with HAIRFA (P < 0.001 for all), except DEV was lower (456 vs. 1396 J/cm3, P < 0.001). The spherical ratio was closer to 1 with HAIRFA (1.23 vs. 1.46). Coagulation diameters, volume, and average power of HAIRFA increased significantly with longer ablation times. While with NSIRFA, these characteristics were stable till later 20 min, except the power decreased with longer ablation times.
Conclusions
HAIRFA creates much larger and more spherical lesions by increasing overall energy deposition, modulating thermal conductivity, and transferring heat during ablation.


Similar content being viewed by others
References
Ruzzenente A, Guglielmi A, Sandri M, Campagnaro T, Valdegamberi A, Conci S, et al. Surgical resection versus local ablation for HCC on cirrhosis: results from a propensity case-matched study. J Gastrointest Surg. 2012;16(2):301–11.
Ayav A, Germain A, Marchal F, Tierris I, Laurent V, Bazin C, et al. Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg. 2010;200(4):435–9.
Tanaka T, Isfort P, Braunschweig T, Westphal S, Woitok A, Penzkofer T, et al. Superselective particle embolization enhances efficacy of radiofrequency ablation: effects of particle size and sequence of action. Cardiovasc Interv Radiol. 2013;36(3):773–82.
Kim YK, Lee JM, Kim SW, Kim CS. Combined radiofrequency ablation and hot saline injection in rabbit liver. Investig Radiol. 2003;38(11):725–32.
Kettenbach J, Kostler W, Rucklinger E, Gustorff B, Hupfl M, Wolf F, et al. Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. Am J Roentgenol. 2003;180(6):1537–45.
Burdio F, Guemes A, Burdio JM, Navarro A, Sousa R, Castiella T, et al. Large hepatic ablation with bipolar saline-enhanced radiofrequency: an experimental study in in vivo porcine liver with a novel approach. J Surg Res. 2003;110(1):193–201.
Lee JM, Rhim H, Han JK, Youn BJ, Kim SH, Choi BI. Dual-probe radiofrequency ablation: an in vitro experimental study in bovine liver. Investig Radiol. 2004;39(2):89–96.
Park JY, Park CY, Lee JM. Estimation of saline-mixed tissue conductivity and ablation lesion size. Comput Biol Med. 2013;43(5):504–12.
Lee JM, Kim YK, Kim SW, Han JK, Kim SH, Choi BI. Combined radiofrequency ablation and acetic acid hypertonic saline solution instillation: an in vivo study of rabbit liver. Korean J Radiol. 2004;5(1):31–8.
Goldberg SN, Ahmed M, Gazelle GS, Kruskal JB, Huertas JC, Halpern EF, et al. Radio-frequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating and coagulation-phantom and porcine liver study. Radiology. 2001;219(1):157–65.
Lee JM, Lee YH, Kim YK, Kim SW, Kim SH, Han JK, et al. Combined treatment of radiofrequency ablation and acetic acid injection: an in vivo feasibility study in rabbit liver. European Radiology. 2004;14(7):1303–10.
Sun YX, Cheng W, Han X, Liu Z, Wang QC, Shao H. In vivo experimental study on the effects of fluid in increasing the efficiency of radiofrequency ablation. Asian Pac J Cancer Prev. 2014;15(14):5799–804.
Weijian F, Zan L, Suhong H, Hongmei Z, Lei Z, Yanjie Z, et al. Destructive effect of percutaneous hydrochloric acid injection therapy for liver cancer–a preliminary experimental and clinical study. Gan to Kagaku Ryoho Cancer Chemother. 2006;33(12):1852–6.
Freeman LA, Anwer B, Brady RP, Smith BC, Edelman TL, Misselt AJ, et al. In vitro thermal profile suitability assessment of acids and bases for thermochemical ablation: underlying principles. J Vasc Interv Radiol. 2010;21(3):381–5.
Luo RG, Fao F, Huang JH, Gu YK, Jiang XY, Huang YJ. Diluted hydrochloric acid generates larger radiofrequency ablation lesions in excised porcine livers. Diagn Interv Radiol. 2013;19(2):145–9.
Burdio F, Navarro A, Berjano EJ, Burdio JM, Gonzalez A, Guemes A, et al. Radiofrequency hepatic ablation with internally cooled electrodes and hybrid applicators with distant saline infusion using an in vivo porcine model. Eur J Surg Oncol. 2008;34(7):822–30.
Cha J, Choi D, Lee MW, Rhim H, Kim YS, Lim HK, et al. Radiofrequency ablation zones in ex vivo bovine and in vivo porcine livers: comparison of the use of internally cooled electrodes and internally cooled wet electrodes. Cardiovas Interv Radiol. 2009;32(6):1235–40.
Li X, Zhang L, Fan W, Zhao M, Wang L, Tang T, et al. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: results in ex vivo porcine livers. Int J hyperth. 2011;27(3):240–8.
Lee DH, Lee JM, Lee JY, Kim SH, Han JK, Choi BI. Radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma: long-term results and prognostic factors in 168 patients with cirrhosis. Cardiovasc Interv Radiol. 2014;37(3):705–15.
Liu CH, Yu CY, Chang WC, Dai MS, Hsiao CW, Chou YC. Radiofrequency ablation of hepatic metastases: factors influencing local tumor progression. Ann Surg Oncol. 2014;21(9):3090–5.
Livraghi T, Lazzaroni S, Meloni F. Radiofrequency thermal ablation of hepatocellular carcinoma. Eur J Ultrasound. 2001;13(2):159–66.
Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58(1):89–97.
Rempp H, Hoffmann R, Roland J, Buck A, Kickhefel A, Claussen CD, et al. Threshold-based prediction of the coagulation zone in sequential temperature mapping in MR-guided radiofrequency ablation of liver tumours. Eur Radiol. 2012;22(5):1091–100.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81371652) and Science and Technology Planning Project of Guangdong Province (No. 2012B031800120).
Author Contribution
Study design: Jin-hua Huang; Reagents preparation: Xiong-ying Jiang and Yang-kui Gu; Ex vivo liver experiments: Jin-hua Huang, Xiong-ying Jiang, and Tian-qi Zhang; Measurement of ablation lesions: Yang-kui Gu and Fei Gao; Collection of the data: Xiong-ying Jiang, Tian-qi Zhang, and Ru-hai Zou; Statistical analysis: Xiong-ying Jiang, Fei Gao, and Ru-hai Zou; Manuscript writing: Xiong-ying Jiang, Tian-qi Zhang, and Jin-hua Huang; Constructive suggestions: Yang-kui Gu, Fei Gao, and Tian-qi Zhang; Final approval of the manuscript: Jin-hua Huang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no any actual or potential conflicts of interest exist.
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Informed Consent
Since our experiments were performed in ex vivo bovine liver, additionally informed consent does not apply.
Rights and permissions
About this article
Cite this article
Jiang, Xy., Gu, Yk., Huang, Jh. et al. Ex Vivo Liver Experiment of Hydrochloric Acid-Infused and Saline-Infused Monopolar Radiofrequency Ablation: Better Outcomes in Temperature, Energy, and Coagulation. Cardiovasc Intervent Radiol 39, 600–605 (2016). https://doi.org/10.1007/s00270-015-1218-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-015-1218-9