Abstract
The crystal structure and crystal chemistry of wardite, ideally NaAl3(PO4)2(OH)4·2H2O, was investigated by single-crystal neutron diffraction (data collected at 20 K) and electron microprobe analysis in wavelength-dispersive mode. The empirical formula of the sample used in this study is: (Na0.91Ca0.01)Σ = 0.92(Al2.97Fe3+0.05Ti0.01)Σ = 3.03(P2.10O8)(OH)4·1.74H2O. The neutron diffraction data confirm that the crystal structure of wardite can be described with a tetragonal symmetry (space group P41212, a = b = 7.0577(5) and c = 19.0559(5) Å at 20 K) and consists of sheets made of edge-sharing Na-polyhedra and Al-octahedra along with vertex-sharing Al-octahedra, parallel to (001), connected by P-tetrahedra and H bonds to form a (001) layer-type structure, which well explains the pronounced {001} cleavage of the wardite crystals. The present data show that four crystallographically independent H sites occur in the structure of wardite, two belonging to a H2O molecule (i.e., H1–O6–H2) and two forming hydroxyl groups (i.e., O5–H3 and O7–H4). The location of the hydrogen atoms allows us to define the extensive network of H bonds: the H atoms belonging to the H2O molecule form strong H bonds, whereas both the H atoms belonging to the two independent hydroxyl groups form weak interactions with bifurcated bonding schemes. As shown by the root-mean-square components of the displacement ellipsoids, oxygen and hydrogen atoms have slightly larger anisotropic displacement parameters compared to the other sites (populated by P, Al and Na). The maximum ratio of the max and min root-mean-square components of the displacement ellipsoids is observed for the protons of the hydroxyl groups, which experience bifurcated H-bonding schemes. A comparative analysis of the crystal structure of wardite and fluorowardite is also provided.


Similar content being viewed by others
References
Archer J, Lehmann MS (1986) A simple adjustable mount for a two-stage cryorefrigerator on an Eulerian cradle. J Appl Crystallogr 19:456–459
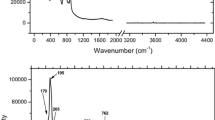
Breitinger DK, Belz HH, Hajba L, Komlosi V, Mink J, Brehm G, Colognesi D, Parker SF, Schwab RG (2004) Combined vibrational spectra of natural wardite. J Mol Struct 706:95–99
Busing WR, Levy HA (1964) The effect of thermal motion on the estimation of bond lengths from diffraction measurements. Acta Crystallogr 17:142–146
Chiari G, Ferraris G (1982) The water molecules in crystalline hydrates studied by neutron diffraction. Acta Crystallogr B 38:2331–2341
Davison JM (1896) Wardite: a new hydrous basic phosphate of alumina. Am J Sci 2:154–155
Duisenberg AJM (1992) Indexing in single-crystal diffractometry with an obstinate list of reflections. J Appl Crystallogr 25:92–96
Fanfani L, Nunzi A, Zanazzi PF (1970) The crystal structure of wardite. Mineral Mag 37:598–605
Frost RL, Erickson KL (2005) Near-infrared spectroscopic study of selected hydrated hydroxylated phosphates. Spectrochim Acta A 61:45–50
Frost RL, Xi YA (2012) Vibrational spectroscopic study of the phosphate mineral Wardite NaAl3(PO4)2(OH)4·2H2O. Spectrochim Acta A93:155–163
Gatta GD, Rotiroti N, McIntyre GJ, Guastoni A, Nestola F (2008) New insights into the crystal chemistry of epididymite and eudidymite from Malosa, Malawi: a single-crystal neutron diffraction study. Am Mineral 93:1158–1165
Gatta GD, McIntyre GJ, Swanson GJ, Jacobsen SD (2012) Minerals in cement chemistry: a single-crystal neutron diffraction and Raman spectroscopic study of thaumasite, Ca3Si(OH)6(CO3)(SO4)·12H2O. Am Mineral l97:1060–1069
Gatta GD, Vignola P, Meven M, Rinaldi R (2013a) Neutron diffraction in gemology: single-crystal diffraction study of brazilianite, NaAl3(PO4)2(OH)4. Am Mineral 98:1624–1630
Gatta GD, Nénert G, Vignola P (2013b) Coexisting hydroxyl groups and H2O molecules in minerals: a single-crystal neutron diffraction study of eosphorite, MnAlPO4(OH)2·H2O. Am Mineral 98:1297–1301
Gatta GD, Jacobsen SD, Vignola P, McIntyre GJ, Guastella G, Abate LF (2014a) Single-crystal neutron diffraction and Raman spectroscopic study of hydroxylherderite, CaBePO4(OH,F). Mineral Mag 78:723–737
Gatta GD, Vignola P, Meven M (2014b) On the complex H-bonding network in paravauxite, Fe2+Al2(PO4)2(OH)2·8H2O: a single-crystal neutron diffraction study. Mineral Mag 78:841–850
Gatta GD, Redhammer GJ, Vignola P, Meven M, McIntyre GJ (2015) Single-crystal neutron diffraction and Mössbauer spectroscopic study of hureaulite, (Mn,Fe)5(PO4)2(HPO4)2(H2O)4. Eur J Mineral 28:93–103
Gatta GD, Fabelo Rosa OR, Fernandez-Diaz MT (2018) On the crystal chemistry of the wardite, NaAl3(PO4)2(OH)4 × 2H2O. Institut Laue-Langevin (ILL, Grenoble). https://doi.org/10.5291/ILL-DATA.5-11-430
Gatta GD, Hålenius U, Bosi F, Cañadillas-Delgado L, Fernandez-Diaz MT (2019) Minerals in cement chemistry: a single-crystal neutron diffraction study of ettringite, Ca6Al2(SO4)3(OH)12·27H2O. Am Mineral. https://doi.org/10.2138/am-2019-6783 (in press)
Heritsch H (1955) Die Raumgruppe von Wardit. Tschermaks Min Petr Mitt 5:246–251
Hurlbut CS (1952) Wardite from beryl mountain, New Hampshire. Am Mineral 37:849–852
Kampf AR, Adams PM, Housley RM, Rossman GR (2014) Fluorowardite, NaAl3(PO4)2(OH)2F2·2H2O, the fluorine analogue of wardite from the Silver Coin mine, Valmy, Nevada. Am Mineral 99:804–810
Larson AC (1967) Inclusion of secondary extinction in least-squares calculations. Acta Crystallogr 23:664–665
Lotti P, Gatta GD, Demitri N, Guastella G, Rizzato S, Ortenzi MA, Magrini F, Comboni D, Guastoni A, Fernandez-Diaz MT (2017) Crystal-chemistry and temperature behavior of the natural hydrous borate colemanite, a mineral commodity of boron. Phys Chem Minerals 45:405–422
Mandarino JA, Sturman BD (1976) Kulanite, a new barium iron aluminum phosphate from the Yukon Territory, Canada. Can Mineral 14:127–131
Mandarino JA, Sturman BD, Corlett MI (1977) Penikisite, the magnesium analogue of kulanite, from the Yukon Territory, Canada. Can Mineral 15:393–395
Matthewman JC, Thompson P, Brown PJ (1982) The Cambridge crystallography subroutine library. J Appl Crystallogr 15:167–171
Moore PB, Araki T, Merlino S, Mellini M, Zanazzi PF (1981) The arrojadite-dickinsonite series, \(\text{KNa}_4\text{Ca}(\text{Fe,Mn})_{14}^{2+}\text{Al}(\text{OH})_2(\text{PO}_4)_{12}\): crystal structure and crystal chemistry. Am Mineral 66:1034–1049
Pouchou J-L, Pichoir F (1991) Quantitative analysis of homogeneous or stratified microvolumes applying the method “PAP”. In: Heinrich KFJ, Newbury DE (eds) Electron probe quantitation. Plenum Press, New York, pp 31–75
Roberts AC, Ansell HG, Jonasson IR, Grice JD, Ramik RA (1986) Rapidcreekite, a new hydrated calcium sulfate–carbonate from the Rapid Creek area, Yukon Territory. Can Mineral 24:51–54
Robertson BT (1982) Occurrence of epigenetic phosphate minerals in a phosphatic iron formation, Yukon Territory. Can Mineral 20:177–187
Robinson GW, Van Velthuizen J, Ansell HG, Sturman BD (1992) Mineralogy of the Rapid Creek and Big Fish river area, Yukon Territory. Mineral Rec 23:1–47
Sears VF (1986) Neutron scattering lengths and cross-sections. In: Sköld K, Price DL (eds) Neutron scattering, methods of experimental physics, vol 23A. Academic Press, New York, pp 521–550
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122
Sheldrick GM (2014) SHELXL-2014. Programs for crystal structure determination and refinement. University of Göttingen, Germany
Steiner T (1998) Opening and narrowing of the water H–O–H angle by hydrogen-bonding effects: re-inspection of neutron diffraction data. Acta Crystallogr B 54:464–470
Sturman BD, Dunn PJ (1984) Garyansellite, a new mineral from Yukon Territory. Am Mineral 69:207–209
Sturman BD, Mandarino JA (1976) Barićite, the magnesium analogue of vivianite, from the Yukon Territory, Canada. Can Mineral 14:403–406
Sturman BD, Mandarino JA, Mrose ME, Dunn PJ (1981) Gormanite, \(\text{Fe}_{3}^{2+}\text{Al}_4(\text{PO}_4)_4(\text{OH})_6\cdot 2\text{H}_{2}\text{O}\), the ferrous analogue of souzalite, and new data for souzalite. Can Mineral 19:381–387
Vassilikou-Dova AB (1993) An EPR study of trivalent iron in wardite. Appl Magn Reson 5:25–29
Wilkinson C, Khamis HW, Stansfield RFD, McIntyre GJ (1988) Integration of single-crystal reflections using area multidetectors. J Appl Crystallogr 21:471–478
Young FG, Robertson BT (1984) The Rapid Creek formation: an Albian Flysch-related phosphatic iron formation in Northern Yukon Territory. In: Stott DF, Glass DJ (eds) The mesozoic of Middle North America. Canadian Society of Petroleum Geologists Memoir 9, Calgary, pp 361–372
Acknowledgements
The authors thank the Institut Laue-Langevin (Grenoble, France), for the allocation of the beamtime and the Institute for Geosciences and Earth Resources of CNR (Padova) for the microprobe analysis of wardite. Two anonymous reviewers and the Editor, M. Rieder, are thanked. This manuscript is dedicated to Pier Francesco Zanazzi (b. 1939), professor of Mineralogy and Crystallography at the University of Perugia (Italy), who solved and refined the structure of wardite with Luca Fanfani and Antonio Nunzi in 1970, on the occasion of his 80th birthday.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatta, G.D., Guastoni, A., Fabelo, O. et al. A single-crystal neutron diffraction study of wardite, NaAl3(PO4)2(OH)4·2H2O. Phys Chem Minerals 46, 427–435 (2019). https://doi.org/10.1007/s00269-018-1013-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-018-1013-7