Abstract
Background
Ward rounds are an essential component of surgical and perioperative care. However, the relative effectiveness of different interventions to improve the quality of surgical ward rounds remains uncertain. The aim of this systematic review was to evaluate the efficacy of various ward round interventions among surgical patients.
Methods
A systematic literature search of the MEDLINE (OVID), EMBASE (OVID), Scopus, Cumulative Index of Nursing and Allied Health (CINAHL), and PsycInfo databases was performed on 7 October 2022 in accordance with PRISMA guidelines. All studies investigating surgical ward round quality improvement strategies with measurable outcomes were included. Data were analysed via narrative synthesis based on commonly reported themes.
Results
A total of 28 studies were included. Most were cohort studies (n = 25), followed by randomised controlled trials (n = 3). Checklists/proformas were utilised most commonly (n = 22), followed by technological (n = 3), personnel (n = 2), and well-being (n = 1) quality improvement strategies. The majority of checklist interventions (n = 21, 95%) showed significant improvements in documentation compliance, staff understanding, or patient satisfaction. Other less frequently reported ward round interventions demonstrated improvements in communication, patient safety, and reductions in patient stress levels.
Conclusions
Use of checklists, technology, personnel, and well-being improvement strategies have been associated with improvements in ward round documentation, communication, as well as staff and patient satisfaction. Future studies should investigate the ease of implementation and long-term durability of these interventions, in addition to their impact on clinically relevant outcomes such as patient morbidity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ward rounds are an essential component of surgical and perioperative care [1]. They allow doctors to communicate with patients, assess progress, and develop treatment plans [2]. The quality of ward rounds may directly impact on patient outcomes [3, 4], with documentation being a key method of communication between clinical teams [1, 5].
Regulatory bodies have provided expected standards of communication and documentation in doctor-patient consultations [6]. Multiple studies have found that documentation during surgical ward rounds consistently fails to achieve these standards [3, 4].Shortfalls may lead to delays in diagnosis, precipitate preventable complications, medicolegal challenges, and ultimately result in worse outcomes for patients [1, 7, 8].
Ward round checklists and proformas have been developed in an attempt to improve patient care through better documentation of patient progress and management plans [9,10,11,12]. Studies have demonstrated improvements in perioperative care through reductions in rates of error and failure to rescue (i.e. death after the development of a postoperative complication), when ward round checklists were utilised [11, 12]. Telerounding and the use of bedside nursing summaries have also been suggested as adjuncts to the standard ward round for surgical patients [13, 14].
Current literature demonstrates a wide variety of different interventions to improve the quality of surgical ward rounds [9, 13,14,15]. However, there is uncertainty surrounding their relative effectiveness, ease of implementation, and impact on patient satisfaction. The objectives of this study were to systematically review and assess the efficacy of previously documented interventions. This may aid in the design and implementation of perioperative quality improvement strategies.
Data sources and search strategy
A systematic literature search of the MEDLINE (OVID), EMBASE (OVID), Scopus, Cumulative Index of Nursing and Allied Health (CINAHL), and PsycInfo databases was performed 7 October 2022. The search string consisted of key words and Medical Subject Headings (MeSH) terms for various surgical specialties (e.g. ‘cardiothoracic’, ‘otorhinolaryngology’, ‘vascular’), medical staff members (e.g. ‘attending’, ‘consultant’, ‘registrar’), and ward rounds (e.g. ‘ward round’, ‘bedside round’, ‘morning round’), among others. These terms were combined using the ‘AND’/‘OR’ Boolean operators (refer to Supplementary Appendix S2 for an exemplar search string using the MEDLINE database).
Databases were searched from their date of inception. The results were restricted to studies published in English. There were no limitations on patient age, geographic location, or study design. Reference lists of included studies and relevant systematic reviews were also hand-searched to identify additional studies for inclusion.
Study selection criteria
All original studies investigating quality improvement strategies implemented during an inpatient surgical ward round were eligible for inclusion. Surgical ward round was defined as any setting or situation in which member(s) of a surgical team assessed patients as part of perioperative care, regardless of surgical specialty. Only studies reporting quality improvement interventions with a measurable outcome on an individual patient (e.g. patient satisfaction, understanding, and/or interpretation of quality of care) or hospital/department (e.g. duration of ward round, time spent per patient, documentation completion rate, and/or percentage of clinical information considered), and those where the majority (> 50%) of included patients were under surgical care, were included.
We excluded case reports (with< 5 patients), articles without an accessible full-text and/or conference abstracts without a full-text publication. Reviews and studies published in languages other than English were also excluded.
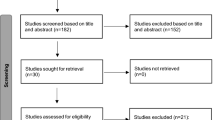
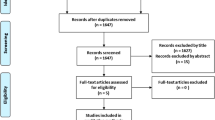
Screening process
Article records from the database searches were exported into EndNote X9 (Clarivate, Philadelphia, PA, USA) and de-duplicated using validated methods [18]. Two investigators (RH, SB) used the Rayyan web application to independently screen titles and abstracts, with relevant full texts then considered for final inclusion [18, 19]. Any discrepancies were addressed through discussion with input from a senior author (CW), until consensus was reached.
Data extraction
Relevant data from included studies were extracted into a proforma Google spreadsheet by a single investigator (RH). These data were independently validated by a second investigator (SB), with any disagreements resolved via mediation with a senior investigator (CW) until consensus was reached. Extracted data comprised study characteristics, conflicts of interest, study funding, surgical specialty, number and designation of medical staff involved, sample size (pre and post-intervention), description of intervention and method of implementation, as well as the comparator intervention. Individual patient and/or hospital/departmental level outcomes were also extracted. Data that were reported in the form of graphs and/or figures were extracted using WebPlotDigitizer (version 4.5; Pacifica, California, USA) [20]. Attempts were made to contact corresponding authors in cases of ambiguous or missing data [21].
Quality assessment
Two authors (RH and SB) independently performed methodological quality assessment of included studies, with disputes resolved through discussion. The Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool [22] and Joanna Briggs Institute (JBI) Critical Appraisal Checklist [23] were used to appraise prospective and retrospective cohort studies, respectively, while the Cochrane Collaboration’s Risk of Bias 2.0 (ROB2) tool was used to assess risk of bias within randomised controlled trials (RCTs) [24]. ROBINS-I results were depicted pictorially using the Risk-of-Bias Visualization (robvis) package in RStudio (R Studio, Boston, MA) [25].
Analysis
Data were analysed via narrative synthesis according to major reported themes among the included studies. Simple descriptive statistics were used to quantitatively report data where possible.
Study characteristics
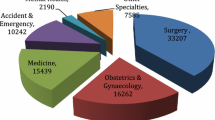
Characteristics of the 28 included studies are provided in Table 1. Other than three studies, the remainder were published within the last decade [21, 31, 35]. Most were cohort studies (n = 25, 89.3%), followed by RCTs (n = 3, 10.7%). Most studies were conducted in the UK (n = 11, 39.3%), followed by the USA (n = 6, 21.4%), and Aotearoa New Zealand (n = 4, 14.3%). All were single-centre studies, including patients from a range of surgical specialties; general (n = 8, 28.6%) and orthopaedic surgery (n = 6, 21.4%), surgical intensive care unit (including general surgical, trauma, and burns patients; n = 3, 10.7%), trauma surgery (n = 3, 10.7%), and urology (n = 3, 10.7%) were the specialties assessed most frequently.
Quality assessment
Quality assessment results using the ROBINS-I tool are depicted in Supplementary Figure S1. Four prospective cohort studies were judged to be at critical risk of bias, principally due to the impact of unmeasured confounding variables [13, 30, 34, 38] Outcomes were measured through valid and reliable means, with sufficiently long follow-up duration, in 12 of the 15 (80%) retrospective cohort studies (Supplementary Appendix S3). However, none of the authors identified or statistically adjusted for any confounding factors in their analyses. The RCTs were mostly at high risk of bias (n = 2 studies, 66.7%) [9, 14], resulting from outcome assessors who were unblinded to the ward round intervention of interest (Supplementary Figures S2–3).
Ward round interventions
A range of quality improvement interventions were implemented during surgical ward rounds. In total, 22 studies used some form of a ward round checklist or proforma (refer to Supplementary Appendix S4 for an example of a ward round checklist). Other interventions included a ‘surgeon of the week’ rounding system (n = 1) [26], additional telerounding on postoperative patients (n = 1) [14], involvement of a specialist radiologist during the ward round (n = 1) [31], digital record keeping (n = 1) [36], mobile tablet use during inpatient services (n = 1) [37], and implementation of active breaks during the ward round (n = 1) [30].
Checklists and proformas served as a guidance for information that should be covered in a surgical ward round, or a template to ensure adequate documentation of essential ward round points. Of the 22 studies that employed a checklist or a proforma, most introduced physical stickers or forms which were placed in a patient’s medical record (82%, 18/22), whereas information printouts displayed throughout the ward were trialled in three studies (Table 2) [34, 44, 46].
Outcomes
Main findings and limitations of included checklist/proforma studies are summarised in Table 2. Supplementary Appendix S5 provides a summary of findings and limitations of all included studies grouped by theme of intervention.
Documentation criteria
Four studies implemented a ‘Plan, Do, Study, Act’ (PDSA) cycle design, whereby ward round interventions were iteratively reviewed and improved after each study [5, 13, 27, 32]. Proforma checklists were used in all of these, in addition to completion of a pre-intervention audit to evaluate baseline documentation compliance against agreed documentation criteria. All studies demonstrated significant improvements in most criteria, such as the documentation of date and time, clinician leading ward round, impression, management plan, and venous thromboembolism (VTE) assessment. Alamri and colleagues [28] reviewed compliance against a proforma sticker utilised in a previous study [27]
Resources and personnel
Yorkgitis et al. [46] introduced a laboratory tests and chest X-ray imaging section on their daily intensive care unit (ICU) checklist. There was no significant difference in the mean number of chest x-rays and coagulation tests requested each day. There was also no significant change in the mean daily number of complete blood counts, chemistry panels, arterial blood gases, and red blood cell transfusions ordered.
Baker et al. [31] reported that presence of a consultant radiologist on the surgical ward round resulted in a significant reduction in the number of nuclear medicine scans, ultrasound scans, body computed tomography (CT) scans, barium enemas, and upper gastrointestinal (GI) series performed. The average hospital length of stay also decreased from 21.4 to 18.4 days. Interestingly, the number of abdominal plain films obtained increased when a consultant radiologist was present.
Staff and patient satisfaction
Pre- and post-intervention surveys were completed by staff and patients to measure satisfaction levels. Generally, ward round quality improvement strategies were well received by staff and patients. Two studies found that checklists had utility as a tool for learning and guiding ward round documentation [3, 21]. Krishnamohan et al. [3] found ward round checklists to be a useful method for deconstructing power hierarchies and encouraging junior team members to ask questions regarding patient care.
Non-checklist interventions also elicited positive responses. Interventions such as the institution of active breaks during the surgical ward round [30], adjunctive telerounding [14], and use of electronic patient records [36] all demonstrated improved staff satisfaction compared to standard surgical ward rounds. In addition, Chaudary and colleagues [36] explored how electronic patient records created extra opportunities for junior staff to learn imaging interpretation techniques amidst the ward round. Abbas et al. [26] concluded that a ‘surgeon of the week’ rounding system was beneficial for both staff and patient satisfaction, and also patient safety and efficiency of the surgical ward round. Following implementation, there were a reduction in the total number of safety complaints, an increase in work relative value units/revenue, and an increase in both employee satisfaction and parental satisfaction in a paediatric surgical unit.
Communication and documentation
Five studies investigated the impact of checklist interventions on communication between staff and patients. Alazzawi et al. [29] reported that all surveyed staff members (n = 10) preferred a proforma to standard ward rounds due to improved clarity of information. Banfield et al. [32] demonstrated improvements in communication and understanding of diagnosis and management plans among junior team members when a proforma was used during the post-acute surgical ward round. Brown et al. [34] observed improvements in patient understanding of their management plans when a surgical communication checksheet was used. Al-Mahrouqi et al. [27] demonstrated that although improvements in ward round documentation were seen with a post-acute ward round proforma, there was no statistically significant impact on nurse certainty of dietary plans, and the number of times surgical teams were contacted. Shaughnessy and colleagues highlighted that patient communication required further improvement, despite a verbal checklist demonstrating improved nursing clarity and reduced plan omissions being used [13]
Surgical ward round efficiency
Significant reductions in overall ward round duration were observed through the use of mobile tablet technology [37] and a ward round checklist [21, 38]. Aydogdu et al. [14] found that adjunctive telerounding did not result in a statistically significant difference in mean ward round time which was consistent with two other studies that employed a ward round proforma [44, 45].
Patient outcomes
Only two studies investigated the impact of ward round interventions on perioperative patient outcomes [1, 3, 14]. Krishnamohan et al. [3] identified that use of a ward round checklist reduced errors in medication prescriptions, antimicrobial administration, fluid balance monitoring, patient observation charts, and the number of venous thromboembolism (VTE) cases diagnosed. Pucher et al. [1] found that general surgery trainees who utilised a ward round checklist committed significantly fewer critical errors compared to standard surgical rounding, with critical errors defined as the ‘failure to adhere to critical processes in the management of postoperative complications’.
Resilience
Few studies described the durability of surgical ward round quality improvement strategies [3, 27, 28]. Results were inconsistent in two studies; Al-Mahrouqi et al. found that compliance was low six months post-intervention (75% proforma usage), whereas Alamri et al. observed comparatively higher compliance with documentation criteria up to two years post-intervention (> 80% completion across most documentation criteria) [27, 28]. In contrast, Krishnamohan et al. observed a mild decrease in compliance with documentation criteria in the three-month period post-intervention, from 79 to 72%.
Discussion
Surgical rounding is an important aspect of perioperative care, with deficiencies in ward round communication and documentation associated with poorer patient outcomes [3, 5, 28, 38, 44]. This systematic review summarised the results from 28 studies which implemented different surgical ward round interventions to improve perioperative care, with significant improvements shown in the quality of documentation and communication during ward rounds. Studies implementing active ward round breaks, telerounding, and digital patient records demonstrated positive feedback from staff and patients. Checklists or proformas were used most frequently to guide ward rounds and were typically associated with significant improvements in ward round documentation. This is consistent with advice from both The Royal College of Physicians and The Royal College of Nursing, who emphasise the utility of checklists in reducing medical errors, establishing rigorous documentation, and promoting cost-effective strategies for punctual discharge [47]. Other studies have also demonstrated the benefit of checklists for patient documentation and communication [3, 28].
Few studies measured the impact of ward round interventions on patient morbidity and mortality. However, implementation of ward round checklists led to significant reductions in prescribing errors and critical errors related to the management of postoperative complications [1, 3]. It was not possible to determine which of these factors were associated with the greatest impact on patient outcomes. This is an important consideration given that quality improvement strategies targeted at ‘high impact’ interventions are likely to result in disproportionately greater improvements in patient morbidity and mortality. The lack of assessment of clinically meaningful outcomes is a missed opportunity in context of the work required to develop ward round tools.
Subjective improvements in staff and patient communication were demonstrated with the use of checklists or proformas during the surgical ward round [13, 27, 29, 32, 34]. Documentation during the ward round is an important means of communication between clinical teams, with improvements in communication shown to mitigate medical errors and improve patient safety and outcomes [2, 9, 38, 48, 49]. Future studies should aim to develop more objective measures of staff and patient communication to improve assessment of different perioperative quality improvement strategies.
Only three studies assessed longitudinal outcomes of their ward round interventions over time [3, 27, 28]. Any successful ward round intervention should be simple and practical to implement, and consider all parties involved in order to achieve long-term engagement and compliance [14, 30, 36]. Further study into the durability of different perioperative ward round interventions would aid understanding of how improvement is maintained, which factors contribute to long-term adherence, and what strategies may overcome barriers of implementation.
Timing and efficiency of the surgical ward round is another consideration, with some staff apprehension about the extra time required to complete quality improvement interventions [50]. However, evidence regarding the impact of perioperative interventions on ward round timing is conflicting. Use of mobile tablets during the ward round led to a significant reduction in the ward round duration, suggesting that digitalisation may reduce time consuming activities such as finding physical notes or leaving the bedside to view investigation results [37]. Some studies found that checklists reduced ward round time [21, 38] possibly because they provided a set ward round structure. This could be useful as checklists provide a comprehensive ward round agenda, thus reducing the risk of omitting important considerations.
There are several limitations to this review. Data were derived from single-centre studies, with short follow-up durations and infrequent reporting of clinically relevant patient outcomes (e.g. morbidity and mortality). The predominance of observational studies (~ 90% of studies) also introduces considerable selection and confounding bias, limiting the reliability of our conclusions. Most studies also used non-validated questionnaires to measure staff and patient satisfaction. The heterogeneity in outcomes and reporting of data between studies made it difficult to perform meaningful quantitative analyses. In addition, potential impacts of the Hawthorne effect (the phenomenon where an individual may alter or change their behaviour when they are aware of being observed) on outcomes was not accounted for in any of the studies [47], which could be contributing to poor long-term durability of some interventions. Finally, ward round checklists or proformas were the most frequently studied intervention, which possibly relates to their relative ease of development and implementation. Thus, the impact of intervention selection bias could not be determined, despite a systematic and broad search of the surgical literature being performed. This suggests that barriers such as the lack of funding and/or resources may exist, ultimately inhibiting transformative interventions from being trialled in the setting of a surgical ward round.
Future research into the impact of different perioperative interventions should focus on larger patient cohorts, longitudinal follow-up of results, and objectively assessing for improvements in clinical outcomes via audit. The clinical and organisational framework for an optimal ward round are also important considerations, with key aspects being communication, early detection of complications, resilience to staff changes, staff well-being, efficiency, and regular auditing of ward round practices.
Conclusion
Different types of ward round interventions have been implemented to improve the quality of patient care during the perioperative period. Use of checklists or proformas, telerounding, mobile tablet use, electronic patient records, a ‘surgeon of the week’ ward rounding system, as well as the introduction of active breaks during ward rounds have been associated with improvements in ward round documentation, communication, and satisfaction among staff and patients. Future studies should specifically investigate whether these different interventions are feasible to maintain in the long term, and their impact on clinically relevant outcomes such as patient morbidity and mortality.
References
Pucher PH, Aggarwal R, Darzi A (2014) Surgical ward round quality and impact on variable patient outcomes. Ann Surg 259(2):222–226
O’Hare JA (2008) Anatomy of the ward round [Internet]. Eur J Int Med 19:309–313. https://doi.org/10.1016/j.ejim.2007.09.016
Krishnamohan N, Maitra I, Shetty VD (2019) The surgical ward round checklist: improving patient safety and clinical documentation. J Multidiscip Healthc 16(12):789–794
Fernando KJ, Siriwardena AK (2001) Standards of documentation of the surgeon-patient consultation in current surgical practice. Br J Surg 88(2):309–312
Duxbury O, Hili S, Afolayan J (2013) Using a proforma to improve standards of documentation of an orthopaedic post-take ward round. BMJ Qual Improv Rep. https://doi.org/10.1136/bmjquality.u200902.w699
Good Medical Practice (2001) The duties of a doctor registered with the general medical council*. Med Educ 35:70–78. https://doi.org/10.1046/j.1365-2923.2001.0350s1070.x
Shetty K, Poo SXW, Sriskandarajah K, Sideris M, Malietzis G, Darzi A et al (2018) “The Longest Way Round Is The Shortest Way Home”: an overhaul of surgical ward rounds. World J Surg 42(4):937–949. https://doi.org/10.1007/s00268-017-4267-1
Thomas J (2009) Medical records and issues in negligence. Indian J Urol 25(3):384–388
Read J, Perry W, Rossaak JI (2021) Ward round checklist improves patient perception of care. ANZ J Surg 91(5):854–859
Dewson D, Eves V, Gaskell R, Hardman A, Akinpelu I, Woodcock E et al (2020) Surgical ward round proforma can improve documentation and efficiency of ward rounds. Postgrad Med J. https://doi.org/10.1136/postgradmedj-2020-139412
Thompson AG, Jacob K, Fulton J, McGavin CR (2004) Do post-take ward round proformas improve communication and influence quality of patient care? Postgrad Med J 80(949):675–676
Pucher PH, Aggarwal R, Qurashi M, Singh P, Darzi A (2014) Randomized clinical trial of the impact of surgical ward-care checklists on postoperative care in a simulated environment. Br J Surg 101:1666–1673. https://doi.org/10.1002/bjs.9654
Shaughnessy L, Jackson J (2015) Introduction of a new ward round approach in a cardiothoracic critical care unit. Nurs Crit Care 20(4):210–218
Aydogdu O, Şen V, Yarimoglu S, Aydogdu C, Bozkurt IH, Yonguc T (2015) 67 The effect of telerounding on postoperative outcomes, patient and surgeon satisfaction rates in the patients who underwent percutaneous nephrolithotomy: a novel practice in urological patient care. Eur Urol Suppl 14:e1163. https://doi.org/10.1016/s1569-9056(15)30066-x
Lépée C, Klaber RE, Benn J, Fletcher PJ, Cortoos PJ, Jacklin A et al (2012) The use of a consultant-led ward round checklist to improve paediatric prescribing: an interrupted time series study. Eur J Pediatr 171(8):1239–1245
Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M et al (2012) The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev 9(1):2
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T (2016) De-duplication of database search results for systematic reviews in endnote. J Med Libr Assoc 104(3):240–243
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5(1):210
Drevon D, Fursa SR, Malcolm AL (2017) Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif 41(2):323–339
Dhillon P, Murphy RKJ, Ali H, Burukan Z, Corrigan MA, Sheikh A et al (2011) Development of an adhesive surgical ward round checklist: a technique to improve patient safety. Ir Med J 104(10):303–305
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetc R et al (2020) Chapter 7: systematic reviews of etiology and risk. JBI Man Evid Synth. https://doi.org/10.46658/jbimes-20-08
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/bmj.l4898
McGuinness LA, Higgins JPT (2021) Risk-of-bias visualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12(1):55–61
Abbas PI, Zamora IJ, Elder SC, Lee TC, Nuchtern JG (2016) Impact of the surgeon of the week system in an academic pediatric surgery practice. J Pediatr Surg 51:634–638. https://doi.org/10.1016/j.jpedsurg.2015.10.044
Al-Mahrouqi H, Oumer R, Tapper R, Roberts R (2013) Post-acute surgical ward round proforma improves documentation. BMJ Qual Improv Rep. https://doi.org/10.1136/bmjquality.u201042.w688
Alamri Y, Frizelle F, Al-Mahrouqi H, Eglinton T, Roberts R (2016) Surgical ward round checklist: Does it improve medical documentation? a clinical review of Christchurch general surgical notes. ANZ J Surg 86(11):878–882
Alazzawi S, Silk Z, Saha UU, Auplish S, Masterson S (2016) A ward round proforma improves documentation and communication. Br J Hosp Med 77:712–716. https://doi.org/10.12968/hmed.2016.77.12.712
Armas M, Aronowitz D, Gaona R, Coppa G, Barrera R (2021) Active breaks initiative during hospital rounds in the surgical ICU to improve wellness of healthcare providers: an observational descriptive study. World J Surg 45(4):1026–1030. https://doi.org/10.1007/s00268-020-05910-7
Baker B, Stein HD (1986) Radiologic consultation: its application to an acute care surgical ward. Am J Roentgenol 147:637–640. https://doi.org/10.2214/ajr.147.3.637
Banfield DA, Adamson C, Tomsett A, Povey J, Fordham T, Richards SK (2018) “Take Ten” improving the surgical post-take ward round: a quality improvement project. BMJ Open Qual 7:e000045. https://doi.org/10.1136/bmjoq-2017-000045
Blucher KM, Dal Pra SE, Hogan J, Wysocki AP (2014) Ward safety checklist in the acute surgical unit. ANZ J Surg 84(10):745–747
Brown OS, Toi TH, Barbosa PR, Pookarnjanamorakot P, Trompeter A (2019) A patient-centred check sheet improves communication on the trauma ward round. Br J Hosp Med 80(8):472–475
Byrnes MC, Schuerer DJE, Schallom ME, Sona CS, Mazuski JE, Taylor BE et al (2009) Implementation of a mandatory checklist of protocols and objectives improves compliance with a wide range of evidence-based intensive care unit practices. Crit Care Med 37(10):2775–2781
Chaudary MI, Zeb J, Arshad F, Sadiq S, Hanif UK, Saleem U et al (2022) Comparison of digital versus conventional documentation of ward round in terms of staff satisfaction, effect on education, and Adherence to British orthopaedic association guidelines. Cureus 14(8):e27598
Crowson MG, Kahmke R, Ryan M, Scher R (2016) Utility of daily mobile tablet use for residents on an otolaryngology head & neck surgery inpatient service. J Med Syst. https://doi.org/10.1007/s10916-015-0419-8
Dolan R, Broadbent P (2016) A quality improvement project using a problem based post take ward round proforma based on the SOAP acronym to improve documentation in acute surgical receiving. Ann West Med Surg 1(5):45–48
Gilliland N, Catherwood N, Chen S, Browne P, Wilson J, Burden H (2018) Ward round template: enhancing patient safety on ward rounds. BMJ Open Qual 7(2):e000170
Koumoullis HD, Shapev M, Wong G, Gerring S, Patrinios G, Depasquale I et al (2020) Improving the quality of the daily ward round in a plastic surgery unit by adapting the SAFE ward round tool of the Royal College of Surgeons of Edinburgh. J Patient Saf Risk Manag 25:233–238. https://doi.org/10.1177/2516043520960572
Ng J, Abdelhadi A, Waterland P, Swallow J, Nicol D, Pandey S et al (2018) Do ward round stickers improve surgical ward round? A quality improvement project in a high-volume general surgery department. BMJ Open Qual 7:e000341. https://doi.org/10.1136/bmjoq-2018-000341
Pitcher M, Lin JTW, Thompson G, Tayaran A, Chan S (2016) Implementation and evaluation of a checklist to improve patient care on surgical ward rounds. ANZ J Surg 86(5):356–360
Talia AJ, Drummond J, Muirhead C, Tran P (2017) Using a structured checklist to improve the orthopedic ward round: a prospective cohort study. Orthopedics. https://doi.org/10.3928/01477447-20170509-01
Tranter-Entwistle I, Best K, Ianev R, Beresford T, McCombie A, Laws P (2020) Introduction and validation of a surgical ward round checklist to improve surgical ward round performance in a tertiary vascular service. ANZ J Surg 90(7–8):1358–1363
Vukanic D, Kelly EG, Cleary SM (2021) Does an orthopedic ward round pro forma improve inpatient documentation? J Patient Saf 17(8):553–556
YorkgitisLoughlin BKJW, Gandee Z, Bates HH, Weinhouse G (2018) Laboratory tests and X-ray Imaging in a surgical intensive care unit: checking the checklist. J Am Osteopath Assoc 118(5):305–309
Royal College of Physicians of London, Royal College of Nursing (Great Britain) (2012) Ward rounds in medicine: principles for best practice: a joint publication of the Royal College of Physicians and the Royal College of Nursing
SharmaPeters SMJ, PICU/NICU Risk Action Group (2013) Safety by DEFAULT”: introduction and impact of a paediatric ward round checklist. Crit Care 17(5):232
Trahan C, Hui AY, Binepal N (2022) Standardization of rounds on a general paediatric ward: implementation of a checklist to improve efficiency, quality of rounds, and family satisfaction. Paediatr Child Health 27(2):111–117
Fourcade A, Blache JL, Grenier C, Bourgain JL, Minvielle E (2012) Barriers to staff adoption of a surgical safety checklist. BMJ Qual Saf 21(3):191–197
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. There are no sources of funding to report for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, R., Bhat, S., Varghese, C. et al. Interventions to Improve Patient Care on Surgical Ward Rounds: A Systematic Review. World J Surg 47, 3159–3174 (2023). https://doi.org/10.1007/s00268-023-07221-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-023-07221-z