Abstract
Background
The effect of organized colorectal cancer (CRC) screening on type of primary treatment remains sparsely investigated. This study evaluated the difference in primary treatment strategy between patients diagnosed with screen-detected (SD-CRC) and non-screen-detected colorectal cancer (NSD-CRC) in a national CRC screening program.
Methods
This was a retrospective national register-based cohort study. Data on patients aged between 50 and 75 years and diagnosed with SD-CRC or NSD-CRC were retrieved from the national colorectal cancer screening database and the Danish Colorectal Cancer Group database. Outcomes related to surgical invasiveness were compared between the two cohorts. Differences were expressed as relative risks using log-binomial generalized linear regression models. UICC stage IV specific outcomes were analyzed using the same method. All analyses were adjusted for sex, age, type of cancer (colonic/rectal), and Charlson comorbidity index.
Results
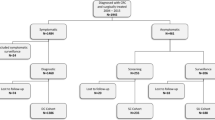
The study included 4707 patients with SD-CRC and 7328 with NSD-CRC. Therapeutic flexible endoscopy (SD-CRC: n = 636 vs. NSD-CRC: n = 334, RR: 2.50, P < 0.001), (robotic-assisted) laparoscopic resection ((n = 616 vs. n = 773, RR: 1.27, P < 0.001), n = 2759 vs. n = 3471, RR: 1.11, P < 0.001), and radical resection (n = 3890 vs. n = 4834, RR: 1.02, P = 0.002) were significantly more frequent in the SD-CRC group. The rates of emergency priority (n = 32 vs. n = 562, RR: 0.09, P < 0.001), open surgery (n = 391 vs. n = 1410, RR: 0.53, P < 0.001), supplementary organ resection (n = 259 vs. n = 860, RR: 0.56, P < 0.001), and stoma formation (n = 526 vs. n = 1040, RR: 0.89, P = 0.007) were significantly lower in the SD-CRC group. The rate of patients undergoing surgery with UICC stage IV disease was significantly higher in the SD-CRC group (SD-CRC: n = 262, NSD-CRC: n = 994, RR: 1.43, P < 0.001).
Conclusion
SD-CRC remained associated with less invasive primary surgical treatment following adjustment for potential healthy user bias. UICC stage IV disease may be less advanced in patients with SD-CRC.

Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Njor SH, Friis-Hansen L, Andersen B et al (2018) Three years of colorectal cancer screening in Denmark. Cancer Epidemiol 57:39–44. https://doi.org/10.1016/j.canep.2018.09.003
Larsen MB, Njor S, Ingeholm P, Andersen B (2018) Effectiveness of colorectal cancer screening in detecting earlier-stage disease—a nationwide cohort study in Denmark. Gastroenterology 155:99–106. https://doi.org/10.1053/j.gastro.2018.03.062
Kubisch CH, Crispin A, Mansmann U et al (2016) Screening for colorectal cancer is associated with lower disease stage: a population-based study. Clin Gastroenterol Hepatol 14:1612-1618.e3. https://doi.org/10.1016/j.cgh.2016.04.008
MacKay C, Ramsay G, Rafferty A, Loudon M (2014) Impact of the Scottish Bowel Cancer Screening Programme on patient and tumour characteristics at a single centre. J Eval Clin Pract 20:7–11. https://doi.org/10.1111/jep.12071
Zorzi M, Fedeli U, Schievano E et al (2015) Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut 64:784–790. https://doi.org/10.1136/gutjnl-2014-307508
Cole SR, Tucker GR, Osborne JM et al (2013) Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust 198:327–330. https://doi.org/10.5694/mja12.11357
Wiltink LM, White K, King MT, Rutherford C (2020) Systematic review of clinical practice guidelines for colorectal and anal cancer: the extent of recommendations for managing long- term symptoms and functional impairments. Support Care Cancer 28:2523–2532. https://doi.org/10.1007/s00520-020-05301-7
Bhama AR, Wafa AM, Ferraro J et al (2016) Comparison of risk factors for unplanned conversion from laparoscopic and robotic to open colorectal surgery using the Michigan surgical quality collaborative (MSQC) database. J Gastrointest Surg 20:1223–1230. https://doi.org/10.1007/s11605-016-3090-6
Zelhart M, Kaiser AM (2018) Robotic versus laparoscopic versus open colorectal surgery: towards defining criteria to the right choice. Surg Endosc 32:24–38. https://doi.org/10.1007/s00464-017-5796-2
Ma Y, Yang Z, Qin H, Wang Y (2011) A meta-analysis of laparoscopy compared with open colorectal resection for colorectal cancer. Med Oncol 28:925–933. https://doi.org/10.1007/s12032-010-9549-5
Cuk P, Kjær MD, Mogensen CB et al (2022) Short-term outcomes in robot-assisted compared to laparoscopic colon cancer resections: a systematic review and meta-analysis. Surg Endosc 36:32–46. https://doi.org/10.1007/s00464-021-08782-7
Sajid MS, Farag S, Leung P et al (2014) Systematic review and meta-analysis of published trials comparing the effectiveness of transanal endoscopic microsurgery and radical resection in the management of early rectal cancer. Color Dis 16:2–14. https://doi.org/10.1111/codi.12474
Silva GLR, de Moura EGH, Bernardo WM et al (2016) Endoscopic versus surgical resection for early colorectal cancer-a systematic review and meta-analysis. J Gastrointest Oncol 7:326–335. https://doi.org/10.21037/jgo.2015.10.02
Hassan C, Repici A, Sharma P et al (2016) Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut 65:806–820. https://doi.org/10.1136/gutjnl-2014-308481
tot Babberich MPMDN, Vermeer NCA, Wouters MWJM et al (2018) Postoperative outcomes of screen-detected vs non–screen-detected colorectal cancer in the netherlands. JAMA Surg 153:e183567. https://doi.org/10.1001/jamasurg.2018.3567
Wilhelmsen M, Njor SH, Roikjær O et al (2021) Impact of screening on short-term mortality and morbidity following treatment for colorectal cancer. Scand J Surg 110:465–471. https://doi.org/10.1177/14574969211019824
Spolverato G, Capelli G, Battagello J et al (2021) More favorable short and long-term outcomes for screen-detected colorectal cancer patients. Front Oncol 11:1–10. https://doi.org/10.3389/fonc.2021.620644
Sebastian E, Courtier R, Macià F et al (2017) The impact of screening on short-term outcome after surgery for colorectal cancer. Rev Esp Enfermedades Dig 109:485–490. https://doi.org/10.17235/reed.2017.4569/2016
Saraste D, Martling A, Nilsson PJ et al (2017) Screening vs. non-screening detected colorectal cancer: differences in pre-therapeutic work up and treatment. J Med Screen 24:69–74. https://doi.org/10.1177/0969141316656216
Shrank WH, Patrick AR, Brookhart MA (2011) Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 26:546–550. https://doi.org/10.1007/s11606-010-1609-1
Von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 4:1623–1627. https://doi.org/10.1371/journal.pmed.0040296
Thomsen MK, Njor SH, Rasmussen M et al (2017) Validity of data in the Danish colorectal cancer screening database. Clin Epidemiol 9:105–111. https://doi.org/10.2147/CLEP.S124454
Green A (2011) Danish clinical databases: an overview. Scand J Public Health 39:68–71. https://doi.org/10.1177/1403494811402413
Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H (2011) Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health 39:12–16. https://doi.org/10.1177/1403494811399956
SAS Institute Inc. (2011) Sas enterprise guide 7.1
Holmgren K, Kverneng Hultberg D, Haapamäki MM et al (2017) High stoma prevalence and stoma reversal complications following anterior resection for rectal cancer: a population- based multicentre study. Color Dis 19:1067–1075. https://doi.org/10.1111/codi.13771
Tong GJ, Zhang GY, Liu J et al (2018) Comparison of the eighth version of the American joint committee on cancer manual to the seventh version for colorectal cancer: a retrospective review of our data. World J Clin Oncol 9:148–161. https://doi.org/10.5306/wjco.v9.i7.148
Amin MB, Greene FL, Edge SB et al (2017) The Eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67:93–99. https://doi.org/10.3322/caac.21388
Vogel JD, Eskicioglu C, Weiser MR et al (2017) The American society of colon and rectal surgeons clinical practice guidelines for the treatment of colon cancer. Dis Colon Rectum 60:999–1017. https://doi.org/10.1097/DCR.0000000000000926
Pagès F, Mlecnik B, Marliot F et al (2018) International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391:2128–2139. https://doi.org/10.1016/S0140-6736(18)30789-X
Peng J, Wang Z, Chen W et al (2010) Integration of genetic signature and TNM staging system for predicting the relapse of locally advanced colorectal cancer. Int J Colorectal Dis 25:1277–1285. https://doi.org/10.1007/s00384-010-1043-1
Zaborowski AM, Winter DC, Lynch L (2021) The therapeutic and prognostic implications of immunobiology in colorectal cancer: a review. Br J Cancer 125:1341–1349. https://doi.org/10.1038/s41416-021-01475-x
Remschmidt C, Wichmann O, Harder T (2015) Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: a systematic review. BMC Infect Dis 15:429. https://doi.org/10.1186/s12879-015-1154-y
Funding
Bispebjerg Hospital Helsefonden: 19-B-0032. The Gangsted Foundation: A35137 Capital Region of Denmark: A6205. The Danish Cancer Research Foundation: FID20823. The Memory Foundation of Inge and Jørgen Larsen: 10537-003 The Memory Foundation of Knud and Edith Eriksen. The Foundation of Aase and Ejnar Danielsen: 21-10-0230 The Dagmar Marshall Foundation. The A.P. Møller Foundation: L-2021-00062 The P.A. Messerschmidt and Wife Foundation Louis-Hansen Foundation: 22-2B-11171 / L 243.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dressler, J., Njor, S.H., Jørgensen, L.N. et al. Less Invasive Primary Treatment for Colorectal Cancer After Implementation of National Screening: A Nationwide Cohort Study. World J Surg 47, 2877–2887 (2023). https://doi.org/10.1007/s00268-023-07142-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-023-07142-x