Abstract
Background
Considering advances in current post-recurrence treatment, we examined the prognostic significance of the number of risk factors for loss-of-exercise capacity (LEC) after lung cancer surgery, which were identified by our previous prospective observational study.
Methods
Risk factors for LEC were defined as a short baseline 6-min walk distance (<400 m), older age (≥75 years), and low predicted postoperative diffusing capacity for carbon monoxide (<60%). Patients were classified as Risk 0/I/II/III according to the number of risk factors. The survival data were retrospectively analyzed.
Results
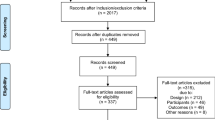
Between 2014 and 2017, 564 patients (n = 307, 193, 57, 7; Risk 0/I/II/III) who underwent lung cancer surgery were included in the study. The number of risk factors was associated with smoking status, predicted postoperative forced expiratory volume in 1 s, histology, pathological stage, and adjuvant therapy. In a multivariate Cox regression analysis, compared to Risk 0, Risk I/II/III showed significant associations with overall survival (hazard ratios: 1.92, 3.35, 9.21; 95% confidence interval: 1.27–2.92, 2.01–5.58, 3.64–23.35; Risk I/II/III, respectively). In 141 patients with recurrence, molecular targeted therapies (MTTs) or immune checkpoint inhibitors (ICIs) were included in 58%, 47%, 32%, and 0% (Risk 0/I/II/III) during the course of treatment. In patients with MTT/ICI treatment, the estimated 1-year and 3-year post-recurrence survival rates were 88% and 58%, respectively.
Conclusions
Risk classification for LEC was associated with survival after lung cancer surgery, as well as post-recurrence treatment. The concept of physical performance-preserving surgery may contribute to improving the outcomes of current lung cancer treatment.


Similar content being viewed by others
Abbreviations
- 6 MWD:
-
6-Minute walk distance
- ADC:
-
Adenocarcinoma
- ALK:
-
Anaplastic lymphoma kinase
- BMI:
-
Body mass index
- BSC:
-
Best supportive care
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DFS:
-
Disease-free survival
- DLCO :
-
Diffusing capacity of the lungs for carbon monoxide
- EGFR:
-
Epidermal growth factor receptor
- FEV1.0 :
-
Forced expiratory volume in one second
- HR:
-
Hazard ratio
- ICI:
-
Immune checkpoint inhibitor
- IQR:
-
Interquartile range
- LEC:
-
Loss-of-exercise capacity
- MTT:
-
Molecular targeted therapy
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death ligand-1
- ppo:
-
Predicted postoperative
- PRS:
-
Post-recurrence survival
- SCC:
-
Squamous cell carcinoma
- TKI:
-
Tyrosine kinase inhibitor
- VATS:
-
Video-assisted thoracoscopic surgery
References
Howington JA, Blum MG, Chang AC et al (2013) Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e278S–e313S
Rivera MP, Mehta AC, Wahidi MM (2013) Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e142S–e165S
Ozeki N, Iwano S, Taniguchi T et al (2014) Therapeutic surgery without a definitive diagnosis can be an option in selected patients with suspected lung cancer. Interact Cardiovasc Thorac Surg 19:830–837
Chen-Yoshikawa TF, Fukui T, Nakamura S et al (2020) Current trends in thoracic surgery. Nagoya J Med Sci 82:161–174
Gonzalez-Rivas D (2016) Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg 5:85–91
Feczko AF, Wang H, Nishimura K et al (2019) Proficiency of robotic lobectomy based on prior surgical technique in the society of thoracic surgeons general thoracic database. Ann Thorac Surg 108:1013–1020
Suzuki K, Saji H, Aokage K et al (2019) Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg 158:895–907
Kobayashi Y, Mitsudomi T (2016) Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci 107:1179–1186
Suresh K, Naidoo J, Lin CT, Danoff S (2018) Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest 154:1416–1423
Wu YL, Tsuboi M, He J et al (2020) Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 383:1711–1723
Brunelli A, Kim AW, Berger KI et al (2013) Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physiciansevidence-based clinical practice guidelines. Chest 143:e166S-e190S
Brunelli A, Charloux A, Bolliger CT et al (2009) ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 34:17–41
Brunelli A, Pompili C, Salati M et al (2014) Preoperative maximum oxygen consumption is associated with prognosis after pulmonary resection in stage I non-small cell lung cancer. Ann Thorac Surg 98:238–242
Ozeki N, Fukui T, Kawaguchi K et al (2018) A survival analysis using physique-adjusted tumor size of non-small cell lung cancer. Int J Clin Oncol 23:266–274
Tanaka S, Ozeki N, Mizuno Y et al (2021) Preoperative paraspinous muscle sarcopenia and physical performance as prognostic indicators in non-small-cell lung cancer. J Cachexia Sarcopenia Muscle 12:646–656
Ozeki N, Fukui T, Iwano S et al (2021) Factors associated with changes in the 12-m stair-climbing time after lung lobectomy. Gen Thorac Cardiovasc Surg 69:282–289
Yılmaz E, Özalevli S, Ersöz H et al (2013) Comparison of health-related quality of life and exercise capacity according to stages in patients with non-small cell lung cancer. Tuberk Toraks 61:131–139
Kushibe K, Kawaguchi T, Kimura M et al (2008) Changes in ventilatory capacity, exercise capacity, and pulmonary blood flow after lobectomy in patients with lung cancer–which lobectomy has the most loss in exercise capacity? Interact Cardiovasc Thorac Surg 7:1011–1014
Salati M, Brunelli A, Xiumè F et al (2017) Video-assisted thoracic surgery lobectomy does not offer any functional recovery advantage in comparison to the open approach 3 months after the operation: a case matched analysis†. Eur J Cardiothorac Surg 51:1177–1182
Bade BC, Thomas DD, Scott JB et al (2015) Increasing physical activity and exercise in lung cancer: reviewing safety, benefits, and application. J Thorac Oncol 10:861–871
Spruit MA, Singh SJ, Garvey C et al (2013) An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 188:e13-64
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. (2002) ATS statement: guidelines for the 6-min walk test. Am J Respir Crit Care Med 166:111–117.
Morley JE, Abbatecola AM, Argiles JM et al (2011) Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 12:403–409
Lee H, Kim HK, Kang D et al (2020) Prognostic value of 6-min walk test to predict postoperative cardiopulmonary complications in patients with non-small cell lung cancer. Chest 157:1665–1673
Wesolowski S, Orlowski TM, Kram M (2020) The 6-min walk test in the functional evaluation of patients with lung cancer qualified for lobectomy. Interact Cardiovasc Thorac Surg 30:559–564
Brierley JD, Gospodarowicz MK, Wittekind C (2016) TNM classification of malignant tumours, 8th edn. Wiley-Blackwell, New York
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG (2015) WHO classification of tumours of the lung, pleura, thymus and heart. IARC Press, Lyon
Ozeki N, Fukui T, Taniguchi T, Usami N, Kawaguchi K, Ito S, Sakao Y, Mitsudomi T, Hirakawa A, Yokoi K (2014) Significance of the serum carcinoembryonic antigen level during the follow-up of patients with completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg 45:687–692
Ferguson MK, Watson S, Johnson E, Vigneswaran WT (2014) Predicted postoperative lung function is associated with all-cause long-term mortality after major lung resection for cancer. Eur J Cardiothorac Surg 45:660–664
Ozeki N, Kawaguchi K, Fukui T, Fukumoto K, Nakamura S, Hakiri S, Kato T, Hirakawa A, Okasaka T, Yokoi K (2017) The diffusing capacity of the lung for carbon monoxide is associated with the histopathological aggressiveness of lung adenocarcinoma. Eur J Cardiothorac Surg 52:969–974
Licker M, Karenovics W, Diaper J et al (2017) Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. 12:323–333
Funding
Nothing to declare.
Author information
Authors and Affiliations
Contributions
NO contributed to conceptualization; NO contributed to methodology; NO contributed to formal analysis; YK, YM, TI, MN, MG, SN and KF contributed to investigation; NO and MG contributed to data curation; NO contributed to writing—original draft preparation; YK, MG, SN, KF and TF.C-Y. contributed to writing—review and editing; TF.C-Y. contributed to supervision; TF.C-Y contributed to project administration.
Corresponding author
Ethics declarations
Conflict of interest
Nothing to declare.
Ethical approval
The Institutional Review Board of our hospital approved this study and waived the requirement for individual patient consent (2020-0375, 02/11/2020).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozeki, N., Kadomatsu, Y., Mizuno, Y. et al. Risk Assessment for Loss-of-Exercise Capacity After Lung Cancer Surgery: Current Advances in Surgery and Systemic Treatment. World J Surg 46, 933–941 (2022). https://doi.org/10.1007/s00268-021-06427-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06427-3