Abstract
Background
Complications are common after colorectal surgery and remain a target for quality improvement. Lower preoperative physical functioning is associated with poor postoperative outcomes, but assessment often relies on subjective judgment or resource-intensive tests. Recent literature suggests that self-reported functional capacity, measured using the Duke Activity Status Index (DASI), is a strong predictor of postoperative outcomes. This study aimed to estimate the extent to which DASI predicts 30-day complications after colorectal surgery.
Methods
In this observational study, 100 patients undergoing colorectal resection [median age 63, 57% men, 81% laparoscopic, 37% rectal surgery] responded to DASI two weeks preoperatively. Complications were classified according to Clavien–Dindo and quantified using the comprehensive complication index (CCI). Our primary analysis targeted the relationship between preoperative DASI and odds of complications. Secondary analyses focused on 30-day severe complications, CCI, readmissions, and length of stay (LOS). We also explored the predictive ability of DASI with scores dichotomized based on a previously validated threshold (≤ 34).
Results
Mean preoperative DASI was 48 ± 12. Forty-six patients (46%) experienced 30-day complications (8% severe, CCI 9.6 ± 15). Lower DASI scores were associated with higher odds of complications (OR 1.08, 95%CI 1.03–1.14; p = 0.001). Preoperative DASI was also an independent predictor of severe complications, CCI, and readmissions. The predictive ability was supported when scores were dichotomized at ≤ 34.
Conclusion
DASI is a significant predictor of postoperative complications after colorectal surgery. This questionnaire can be easily implemented in clinical practice to identify patients with low preoperative functional capacity and target interventions to those at higher risk.
Similar content being viewed by others
References
Archer S, Pinto A, Vuik S et al (2019) Surgery, complications, and quality of life: a longitudinal cohort study exploring the role of psychosocial factors. Ann Surg 270:95–101. https://doi.org/10.1097/SLA.0000000000002745
Brown SR, Mathew R, Keding A et al (2014) The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg 259:916–923. https://doi.org/10.1097/SLA.0000000000000407
Khuri SF, Henderson WG, DePalma RG et al (2015) Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 242:326–343
Birkmeyer JD, Gust C, Dimick JB et al (2012) Hospital quality and the cost of inpatient surgery in the United States. Ann Surg 255:1–5. https://doi.org/10.1097/SLA.0b013e3182402c17
Vonlanthen R, Slankamenac K, Breitenstein S et al (2011) The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 254:907–913. https://doi.org/10.1097/SLA.0b013e31821d4a43
Schilling PL, Dimick JB, Birkmeyer JD (2008) Prioritizing quality improvement in general surgery. J Am Coll Surg 207:698–704. https://doi.org/10.1016/j.jamcollsurg.2008.06.138
Scarborough JE, Schumacher J, Kent KC et al (2017) Associations of specific postoperative complications with outcomes after elective colon resection: a procedure-targeted approach toward surgical quality improvement. JAMA Surg 152:e164681
Marres CCM, van de Ven AWH, Verbeek PCM et al (2016) The effect of a postoperative quality improvement program on outcomes in colorectal surgery in a community hospital. Int J Colorectal Dis 31:1603–1609. https://doi.org/10.1007/s00384-016-2619-1
Biccard BM (2005) Relationship between the inability to climb two flights of stairs and outcome after major non-cardiac surgery: implications for the pre-operative assessment of functional capacity. Anaesthesia 60:588–593. https://doi.org/10.1111/j.1365-2044.2005.04181.x
Fleisher LA, Fleischmann KE, Auerbach AD et al (2014) 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 130:2215–2245. https://doi.org/10.1161/CIR.0000000000000105
Kristensen SD, Knuuti J, Saraste A et al (2014) 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European society of cardiology (ESC) and the European society of anaesthesiology (ESA). Eur Heart J 35:2383–2431
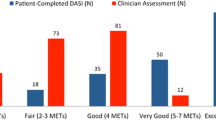
Melon CC, Eshtiaghi P, Luksun WJ et al (2014) Validated questionnaire vs physicians’ judgment to estimate preoperative exercise capacity. JAMA Intern Med 174:1507–1508
Stokes JW, Wanderer JP, McEvoy MD (2016) Significant discrepancies exist between clinician assessment and patient self-assessment of functional capacity by validated scoring tools during preoperative evaluation. Perioper Med (Lond) 5:18
Wijeysundera DN, Pearse RM, Shulman MA et al (2018) Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 391:2631–2640
Richardson K, Levett DZH, Jack S et al (2017) Fit for surgery? Perspectives on preoperative exercise testing and training. Br J Anaesth 119:i34–i43
Levett DZH, Jack S, Swart M et al (2018) Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth 120:484–500. https://doi.org/10.1016/j.bja.2017.10.020
Sinclair RCF, Batterham AM, Davies S et al (2012) Validity of the 6 min walk test in prediction of the anaerobic threshold before major non-cardiac surgery. Br J Anaesth 108:30–35. https://doi.org/10.1093/bja/aer322
Lee L, Schwartzman K, Carli F et al (2013) The association of the distance walked in 6 min with pre-operative peak oxygen consumption and complications 1 month after colorectal resection. Anaesthesia 68:811–816. https://doi.org/10.1111/anae.12329
ATS committee on proficiency standards for clinical pulmonary function laboratories, (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166:111–117. https://doi.org/10.1164/ajrccm.166.1.at1102
American Thoracic Society; American College of Chest Physicians (2003) ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167:211–277. https://doi.org/10.1164/rccm.167.2.211
Hlatky MA, Boineau RE, Higginbotham MB et al (1989) A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 64:651–654. https://doi.org/10.1016/0002-9149(89)90496-7
Hanley C, Wijeysundera DN, Duminda N (2021) Perioperative risk assessment - focus on functional capacity. Curr Opin Anaesthesiol 34(3):309–316. https://doi.org/10.1097/ACO.0000000000000988
Wijeysundera DN, Beattie WS, Hillis GS et al (2020) Integration of the duke activity status index into preoperative risk evaluation: a multicentre prospective cohort study. Br J Anaesth 124:261–270. https://doi.org/10.1016/j.bja.2019.11.025
Shulman MA, Cuthbertson BH, Wijeysundera DN et al (2019) Using the 6-minute walk test to predict disability-free survival after major surgery. Br J Anaesth 122:111–119. https://doi.org/10.1016/j.bja.2018.08.016
Fiore JF Jr, Castelino T, Pecorelli N et al (2017) Ensuring early mobilization within an enhanced recovery program for colorectal surgery: a randomized controlled trial. Ann Surg 266:223–231. https://doi.org/10.1097/SLA.0000000000002114
Lee L, Mata J, Ghitulescu GA et al (2015) Cost-effectiveness of enhanced recovery versus conventional perioperative management for colorectal surgery. Ann Surg 262:1026–1033. https://doi.org/10.1097/SLA.0000000000001019
Gustafsson UO, Scott MJ, Schwenk W et al (2013) Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS(®)) society recommendations. World J Surg 37:259–284. https://doi.org/10.1007/s00268-012-1772-0
Lassen K, Soop M, Nygren J et al (2009) Consensus review of optimal perioperative care in colorectal surgery: enhanced recovery after surgery (ERAS) group recommendations. Arch Surg 144:961–969. https://doi.org/10.1001/archsurg.2009.170
Von Elm E, Altman DG, Egger M et al (2007) The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147(8):573–577. https://doi.org/10.7326/0003-4819-147-8-200710160-00010
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Slankamenac K, Graf R, Barkun J et al (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258:1–7. https://doi.org/10.1097/SLA.0b013e318296c732
Hendry PO, Hausel J, Nygren J et al (2009) Determinants of outcome after colorectal resection within an enhanced recovery programme. Br J Surg 96:197–205. https://doi.org/10.1002/bjs.6445
Kirchhoff P, Clavien PA, Hahnloser D (2010) Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg 4:5. https://doi.org/10.1186/1754-9493-4-5
Lee CHA, Kong JC, Ismail H et al (2018) Systematic review and meta-analysis of objective assessment of physical fitness in patients undergoing colorectal cancer surgery. Dis Colon Rectum 61(3):400–409. https://doi.org/10.1097/DCR.0000000000001017
Clavien PA, Vetter D, Staiger RD et al (2017) The comprehensive complication index (CCI®): added value and clinical perspectives 3 years “down the line.” Ann Surg 265:1045–1050. https://doi.org/10.1097/SLA.0000000000002132
Banugo P, Amoako D (2017) Prehabilitation. BJA. Education 17:401–405
Fry BT, Hallway A, Englesbe MJ (2018) Moving toward every patient training for surgery. JAMA Surg 153:1089. https://doi.org/10.1001/jamasurg.2018.1658
Wynter-Blyth V, Moorthy K (2017) Prehabilitation: preparing patients for surgery. BMJ 358:j3702
Carli F, Bousquet-Dion G, Awasthi R et al (2020) Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg 155:233–242. https://doi.org/10.1001/jamasurg.2019.5474
Hijazi Y, Gondal U, Aziz O (2017) A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg 39:156–162. https://doi.org/10.1016/j.ijsu.2017.01.111
Thomas G, Tahir MR, Bongers BC et al (2019) Prehabilitation before major intra-abdominal cancer surgery: a systematic review of randomised controlled trials. Eur J Anaesthesiol 36:933–945. https://doi.org/10.1097/EJA.0000000000001030
Riedel B, Li MHG, Lee CHA et al (2021) A simplified (modified) duke activity status index (M-DASI) to characterise functional capacity: a secondary analysis of the measurement of exercise tolerance before surgery (METS) study. Br J Anaesth 126:181–190. https://doi.org/10.1016/j.bja.2020.06.016
Acknowledgements
The authors thank Tanya Castelino (Department of Surgery, McGill University), Petru Niculiseanu (Steinberg-Bernstein Centre for Minimally Invasive Surgery, McGill University), Julia Munden (Department of Anesthesia, McGill University), Meagan Barrett-Bernstein (Department of Anesthesia, McGill University), Enrico M. Minnella (Department of Anesthesia, McGill University), and Berson Augustin (Department of Anesthesia, McGill University) for their assistance with patient recruitment and follow-up. We also acknowledge Pepa Kaneva (Steinberg-Bernstein Centre for Minimally Invasive Surgery, McGill University) for her administrative and technical support during the study.
Funding
This study was supported by a MITACS Elevate Postdoctoral Fellowship (Ref. IT02887). The funder had no role in the design, data collection and analysis, preparation or decision to publish the manuscript. Dr. Fiore Jr. reported receiving research funding from Merck and receiving honorarium as a research consultant to Shionogi. Dr. Feldman reported receiving research funding from Merck and Johnson & Johnson and an educational grant from Medtronic. Dr. Lee reported receiving research funding from Johnson & Johnson. No other authors reported relevant disclosures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The institutional review board approved the original study (MUHC Research Ethics Board ref. 13–329-SDR).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El-Kefraoui, C., Rajabiyazdi, F., Pecorelli, N. et al. Prognostic value of the Duke Activity Status Index (DASI) in patients undergoing colorectal surgery. World J Surg 45, 3677–3685 (2021). https://doi.org/10.1007/s00268-021-06256-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06256-4