Abstract
Background
To assess the impact of primary tumor resection (PTR) on survival and morbidity in incurable colorectal cancer.
Methods
Systematic literature review and meta-analysis to compare PTR versus primary tumor intact (PTI).
Results
Seventy-seven studies were included, reporting on 159,991 participants (94,745 PTR; 65,246 PTI). PTR improved overall survival (hazard ratio [HR] 0.59, P < 0.0001; mean difference [MD] 7.27 months, P < 0.0001), cancer-specific survival (HR 0.47, MD 10.80), and progression-free survival (HR 0.76, MD 1.67). Overall survival remained significantly improved during subgroup analysis of asymptomatic patients (HR 0.69, MD 3.86), elderly patients (HR 0.46, MD 7.71), patients diagnosed after 2000 (HR 0.62, MD 7.29), patients with colon (HR 0.58, MD 6.31) or rectal (HR 0.54, MD 6.88) primary tumor, patients undergoing resection of primary tumor versus non-resectional surgery (NRS) to treat primary tumor complications (HR 0.56, MD 8.72), and of studies with propensity score analysis (HR 0.65, MD 5.68). Overall survival per treatment strategy was: [PTI/chemotherapy] 14.30 months, [PTI/bevacizumab] 17.27 months, [PTR/chemotherapy] 21.52 months, [PTR/bevacizumab] 27.52 months. PTR resulted in 4.5% perioperative mortality and 22.4% morbidity (major adverse events 10.2%, minor 18.5%, reoperation 2.5%, intraabdominal collection/sepsis 2.2%). PTI had 21.7% morbidity (obstruction 14.4%, anemia 11.0%, hemorrhage 1.5%, perforation 0.6%, adverse events requiring surgery 15.8%). NRS resulted in 10.6% perioperative mortality and 21.7% morbidity (major 7.9%, minor 21.7%, reoperation 0.1%).
Conclusions
PTR in patients with incurable colorectal cancer results in a limited improvement of survival without a significant increase in morbidity. PTR should be considered by the multidisciplinary team on an individual patient basis.
Similar content being viewed by others
Introduction
At the time of diagnosis, approximately 20–25% of patients with colorectal cancer presented with synchronous metastases, which are unresectable in 75–90% of these patients [1,2,3]. In addition, patients may present with advanced localized disease that is unresectable due to extensive involvement of surrounding structures or due to involvement of vital structures. For patients with incurable colorectal cancer, an important question which remains unanswered to date is whether the best treatment strategy is primary tumor resection (PTR) with chemotherapy or immediate chemotherapy without PTR. Previous published comparative studies reported conflicting results on this issue, with some studies demonstrating improved survival with PTR compared to primary tumor intact (PTI) [2, 4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32], while other studies found no significant differences between the two groups [33,34,35,36,37,38,39,40], and other studies suggested systemic chemotherapy without resection of the primary tumor is the treatment strategy of choice for patients with incurable colorectal cancer [41,42,43,44,45,46,47,48,49,50]. The purpose of the present study was to perform a systematic review of the literature and employ meta-analytical techniques to compare survival and adverse events in patients undergoing PTR versus PTI, with or without chemotherapy, in order to determine whether PTR should be performed in patients with incurable localized or metastatic colorectal cancer.
Materials and Methods
Search Strategy
This systematic review and meta-analysis was based on a written protocol and was reported in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [51] and Assessing the methodological quality of systematic reviews (AMSTAR) guidelines [52]. A comprehensive literature search was performed of the following databases: PubMed, MEDLINE, Embase, Science Citation Index Expanded, and Cochrane Central Register of Controlled Trials (CENTRAL). Detailed search strategy is provided in the Supplementary Table 1. All abstracts, studies, and citations identified were reviewed, and the references of the identified studies were also searched. No restrictions were made based on language, publication year, or publication status. The latest date for this search was May 2, 2018.
Selection Criteria
Prospective or retrospective studies were considered for this meta-analysis if they met the following criteria:
-
1.
Reported on patients with incurable metastatic colorectal cancer. Incurable metastatic colorectal cancer was defined as the presence of unresectable metastases, including liver metastases, lung metastases, intraperitoneal, and omental carcinomatosis, considered too extensive to achieve a complete or macroscopically curative resection.
-
2.
Reported on patients with incurable advanced localized tumor that was unresectable due to extensive involvement of surrounding structures or due to involvement of vital structures.
-
3.
Reported on survival between patients undergoing PTR versus PTI. The PTI group included patients who may have received chemotherapy, and/or undergone non-resectional surgery (NRS) such as construction of diverting stoma or bypass procedure without resection of the primary tumor, or received no treatment at all.
-
4.
If two studies from the same institution or database reported the same outcomes of interest, only the most recent publication was included in the analysis, unless the studies were mutually exclusive or the outcome was measured at different time intervals.
Outcomes of Interest
Primary outcome:
-
1.
Overall survival
Secondary outcomes:
-
1.
Cancer-specific survival, progression-free survival.
-
2.
Morbidity reported in detail and as major or minor adverse events. A major adverse event was defined as any event that is life-threatening, requires inpatient hospitalization, results in a single organ or multi-organ failure, or requires operative, endoscopic, or radiological intervention to treat it. Major adverse events correspond to Grade III and Grade IV of the Clavien-Dindo classification, and in cases where the authors did not specifically classify the severity of adverse events, this classification method was followed [53, 54].
Two review authors (CS and EK) independently determined the eligibility of all retrieved studies and extracted the required data from the included studies. The risk of bias of the included studies was assessed based on the following bias risk domains: allocation sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and vested interest bias [55]. For each of these risk domains of bias, the studies were categorized as high, low, or uncertain risk.
Statistical Analysis
The mean overall survival in months and the proportion of adverse events, with 95% confidence intervals (CI), by treatment strategy was calculated using the random-effects model [56] in OpenMetaAnalyst [57]. Survival between PTR and PTI was compared as a time-to-event outcome in the form of a hazard ratio (HR) or as a difference in duration of survival in months in the form of mean difference (MD). If the HR was not reported in the publications and survival data were presented in the form of Kaplan–Meier curves, the survival rates at specified times were extracted from the Kaplan–Meier curves to reconstruct the HR estimate and its variance, using methods described by Parmar et al. [58]. The generic inverse variance random-effects model was used [56, 59] in RevMan (Review Manager) version 5.3 (The Nordic Cochrane Center, Copenhagen; The Cochrane Collaboration, 2008) [60]. Publication bias was assessed by graphical exploration with funnel plots, with the absence of publication bias indicated by data points forming a symmetric funnel-shaped distribution. Inter-study heterogeneity (HG) was assessed by graphical exploration with forest plots, by using the Chi2 (or χ2) test, by means of the inconsistency index (I2) to quantify HG, and by performing subgroup analyses [61,62,63].
Results
Eligible Studies
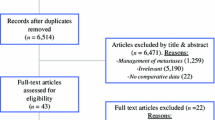
A total of 1416 references were identified through electronic searches of Science Citation Index Expanded (n = 435), EMBASE (n = 17), MEDLINE (n = 943), and CENTRAL (n = 21). Further 34 studies were identified from the references of the above studies. The duplicates between databases were 407 and were excluded. Further, 871 clearly irrelevant references were excluded through screening titles and reading abstracts. The 172 remaining studies were investigated in full text detail, and further 95 studies were excluded. Among these excluded studies, eight were excluded because of duplication of all their reported outcomes of interest in other publications from the same institution or database [9, 28, 29, 64,65,66,67,68]. Figure 1 shows the study flow diagram. Seventy-seven comparative studies fulfilled the inclusion criteria of this meta-analysis [2, 4,5,6,7,8, 10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50, 69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100]. There were 159,991 patients for analysis, including 94,745 (59.2%) in the PTR group and 65,246 (40.8%) in the PTI group. The characteristics of the included studies are shown in Supplementary Table 2. The risk of bias in the included studies is summarized in Supplementary Figure 1 and the risk of bias for each included study is shown in Supplementary Figure 2. Most included studies were retrospective, and on quality assessment they were found to have high risk of bias in the domains of random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessors [55].
Survival
Table 1 demonstrates the mean overall survival in months by treatment strategy. The calculated mean survival by treatment strategy, in order of increasing survival, was as follows: PTI without chemotherapy 4.02 months (95% confidence interval 2.81–5.23 months), PTR without chemotherapy 7.42 months (3.96–10.87), PTI and chemotherapy 14.30 months (12.56–16.05), PTI and chemotherapy with bevacizumab 17.27 months (15.61–18.94), PTR and chemotherapy 21.52 months (19.82–23.22), PTR and chemotherapy with bevacizumab 27.52 months (21.89–33.14).
Table 2 shows the results of the overall meta-analysis and subgroup analysis for survival. Supplementary Table 3 includes additional information such as the results of both fixed-effect and random-effects models, and the tests for heterogeneity (I2 and χ2 test P values). Overall analysis revealed that patients treated with PTR had significantly increased overall survival (HR 0.58, P < 0.0001; Supplementary Figure 3) by 7.46 months (MD 7.46 months, P < 0.0001; Supplementary Figure 4) compared to patients that had PTI, with significant heterogeneity identified between studies (HG P < 0.0001). Similarly, PTR resulted in significantly longer cancer-specific survival (HR 0.44, P < 0.0001; MD 10.01, P < 0.0001), and progression-free survival (HR 0.76, P < 0.0001; MD 1.67, P < 0.0001) compared to PTI, with no significant heterogeneity between studies.
Overall survival remained significantly improved after PTR compared to PTI during subgroup analyses of:
-
patients with metastatic (stage IV) disease (HR 0.60, P < 0.0001) by 7.23 months (MD 7.23, P < 0.0001)
-
studies recruiting patients from 2000 onwards (HR 0.62, P < 0.0001; MD 7.29, P < 0.0001)
-
asymptomatic patients (HR 0.69, P = 0.002; MD 3.86, P = 0.002)
-
studies which performed propensity score-matched analysis (HR 0.65, P = 0.003; MD 5.68, P = 0.0003)
-
elderly patients (aged 65 and older) (HR 0.46, P < 0.0001; MD 7.71, P < 0.0001)
-
colon cancer (HR 0.58, P = 0.01; MD 6.31, P = 0.0005)
-
rectal cancer (HR 0.54, P = 0.0009; MD 6.88, P < 0.0001)
-
comparison of patients after PTR with patients after NRS (HR 0.56, P < 0.0001; MD 8.72, P < 0.0001)
-
patients who did not receive chemotherapy (HR 0.63, P = 0.002), although no significant difference in the duration of survival was demonstrated (MD 3.52, P = 0.09)
-
patients receiving chemotherapy (HR 0.59, P < 0.0001; MD 6.81, P < 0.0001)
-
patients receiving bevacizumab (HR 0.59, P = 0.005; MD 10.56, P = 0.01)
Patients who have undergone PTR and chemotherapy had significantly longer overall survival compared to patients undergoing only PTR without chemotherapy (HR 0.54, P < 0.0001; MD 11.46, P < 0.0001). Similarly, patients in the PTI group who received chemotherapy had significantly improved survival compared to patients in the PTI group who did not receive chemotherapy (HR 0.59, P < 0.0001; MD 5.04, P = 0.001).
Adverse Events
Table 3 shows the proportion of adverse events for the patients in the PTI group and the perioperative adverse events in the NRS subgroup. The total morbidity of the patients in the PTI group was 21.7% (14.9–28.4%), and specifically the most common reported adverse events were: obstruction 14.4%, anemia 11.0%, hemorrhage 1.5%, perforation 0.6%, and fistula 0.3%. The proportion of patients in the PTI group requiring surgery due to adverse events was 15.8% (9.0–22.5%).
Patients belonging to the NRS subgroup (i.e., patients with the primary tumor intact but had stoma diversion or bypass surgery) experienced a 30-day mortality rate of 10.6% (6.5–14.7%) and a morbidity rate of 21.7% (13.8–29.6%). In the same group, the major adverse events rate was 7.9% (2.4–13.4%) and 21.7% (16.2–27.2%) for minor adverse events. There was a 0.1% reoperation rate (0–2.4%). The most common perioperative adverse events in the NRS subgroup were respiratory 3.0%, hemorrhage 2.4%, cardiac 2.3%, ileus/bowel obstruction 1.9%, urinary 1.7%, and deep venous thrombosis/pulmonary embolism 1.0%.
Table 4 shows the proportion of perioperative adverse events of the patients in the PTR group. The 30-day mortality rate related to PTR was 4.5% (3.1–5.9%) and morbidity was 22.4% (17.9–26.8%). The major adverse events rate was 10.2% (7.4–13.0%) and 18.5% for minor adverse events (14.1–22.9). The reoperation rate was 2.5% (1.5–3.5%). The most common perioperative adverse events in the PTR group were wound infection 5.7%, ileus/bowel obstruction 4.0%, urinary 3.7%, respiratory 2.9%, intraabdominal collection/sepsis 2.2%, cardiac 1.9%, anastomotic leak 1.6%, hemorrhage 1.1%, wound dehiscence 0.7%, deep venous thrombosis/pulmonary embolism 0.6%, and cerebrovascular accident 0.3%.
Discussion
This is the largest systematic review and meta-analysis published on this subject to date. Although the overall meta-analysis demonstrated a large effect of PTR in overall survival, due to the large variation in the effect of PTR in survival during subgroup analysis and the associated risk of bias of the included studies, the present review suggested only a limited improvement in survival and that PTR should be considered by the multidisciplinary team on an individual patient basis. Despite published study protocols [3, 101,102,103,104], no randomized controlled trials have been completed to date comparing PTR and PTI in patients with incurable colorectal cancer, and this is partly due to the difficulties in recruiting patients and in designing and performing such trials. Non-randomization of the included studies may have led to patient selection bias and confounders affecting the comparability between the groups at baseline. Concerns were raised that patients who underwent resection of the primary tumor had a more favorable performance status and better overall prognosis in terms of fewer metastatic sites involved, fewer liver-only metastases, and fewer rectal cancer primary tumors [105,106,107]. In addition, some studies included in their PTR groups symptomatic patients who presented with primary tumor-related symptoms or complications at initial presentation [21, 94, 108]. A meta-analysis can address the above limitations by allowing the evaluation of the effects in subsets of patients [109] through the use of prespecified subgroup or sensitivity analyses. In the current meta-analysis, the overall survival remained significantly improved with PTR compared to PTI during subgroup analysis of only patients with metastatic (stage IV) disease, elderly patients aged 65 and older, patients with primary colon cancer or primary rectal cancer, and more recent studies with patients recruited after 2000. To address selection bias, some of the included studies conducted propensity score-matched analysis accounting, among other factors, for number and site of metastases [14, 15, 18, 20, 21, 25, 32, 40, 41], and subgroup analysis of only these studies again demonstrated significant improvement in overall survival with resection of the primary tumor, albeit with a slightly lower survival advantage of 5.68 months. The survival benefit was found to be also reduced in the subgroup analysis of asymptomatic patients (3.86 months), suggesting decreased benefit in this subset of patients, which should be taken into consideration when deciding which patients should proceed with PTR.
With advances in chemotherapy, the response rates of patients with metastatic colorectal cancer to systemic chemotherapy have significantly improved. In contrast to the response rates of approximately 15% to fluorouracil (5-FU) with leucovorin (folinic acid), combinations with other chemotherapeutic agents such as oxaliplatin or irinotecan have yielded response rates of 35–56% [110,111,112,113]. The addition of targeted biotherapy agents targeting vascular endothelial growth factor (anti-VEGF, e.g., bevacizumab) or epidermal growth factor receptor (anti-EGFR in the setting of KRAS wild-type tumors, e.g., cetuximab) to the above combinations have increased response rates further, resulting in significantly improved overall survival and quality of life for patients with incurable metastatic colorectal cancer [111, 112, 114,115,116,117,118]. This meta-analysis has demonstrated that PTR resulted in significantly improved overall survival compared to PTI in the subgroup analysis of only patients that received chemotherapy with increase in overall survival of 7.27 months and especially in the subgroup analysis of only patients that received bevacizumab with increase in survival of 10.56 months. Interestingly, subgroup analysis of patients that did not receive chemotherapy demonstrated only 3.52 months increase in overall survival with PTR compared to PTI without statistical significance (P = 0.09), which suggests that the survival advantage of PTR becomes more pronounced with the use of chemotherapy, and even more so with the use of targeted biotherapy. It is noted that the different chemotherapy regimens and target agents used in combination with PTR or PTI may have a greater impact on oncologic outcomes and patient survival rather than the resection of the primary tumor itself.
Palliative PTR may be required due to adverse events linked to the primary tumor, such as obstruction, perforation or intractable bleeding, but in the setting of current effective chemotherapy regimens, the risk of primary tumor-related complications and the need of subsequent urgent intervention are lower than before. The current meta-analysis identified a 21.7% morbidity rate in the PTI group and a 15.8% risk of emergency surgery due to adverse events. These risks of PTI should be counterweighted against the risks of surgery. It has been suggested that PTR is associated with significant surgical trauma and perioperative mortality and morbidity which may preclude early initiation of systemic therapy [21, 30, 36, 37, 49, 81, 119] or result in a significant systemic inflammatory response and disturbance of homeostasis which may lead to immunosuppression and faster growth of metastases [120]. This study calculated the risk of perioperative mortality to be 4.5% and the risk of morbidity to be 22.4%. Studies which performed multivariate logistic regression analysis to determine independent prognostic variables associated with postoperative mortality and morbidity in patients with stage IV colorectal cancer suggested that baseline characteristics (age, performance status, comorbidity, ASA score), tumor burden (advanced local and metastatic disease), emergency surgery, and primary rectal cancer were related to postoperative morbidity and mortality [49, 105, 119, 121]. An alternative treatment strategy which would prevent complications related to the primary tumor and would theoretically allow the patient to proceed more safely and faster to chemotherapy would be non-resectional surgery (NRS) in the form of a diverting stoma or a bypass procedure. The morbidity related to this treatment strategy was found to be 21.7%. Comparison of the overall survival between PTR and NRS demonstrated that the improvement in survival with PTR remained significant, suggesting that the survival benefit of PTR is not only through the prevention of complications related to the primary tumor.
Although it is not clear why PTR is associated with better outcomes in patients with incurable colorectal cancer, the improvement in overall survival and especially cancer-specific survival following PTR may be attributed to a better response to chemotherapy after reduction of systemic tumor burden. This may explain the improved survival of PTR when combined with chemotherapy. Similar survival benefit has been demonstrated by resecting primary renal and ovarian tumors in the presence of metastatic disease [122,123,124]. Also, based on the ‘seed and soil theory’ which is used to explain the metastatic preference of cancer cells for specific organs, the primary tumor may induce in distant organs a prosperous environment to enhance the growth of metastatic deposits and progression from micro- to macrometastases [125, 126]. Van der Wal et al. suggested that in the presence of the primary tumor, the liver parenchyma adjacent to synchronous liver metastases provides an angiogenic prosperous environment for metastatic tumor growth [127]. Furthermore, Holzel et al. suggested that all distant metastases are initiated before removal of the primary tumor and that metastases do not metastasize again [128]. Based on the results of the current study, every patient with incurable colorectal cancer should be considered for resection of the primary tumor. Nevertheless, not every patient will be a candidate for surgery, and among patients, the benefits of surgery will be different. Studies which performed multivariate analysis to determine which factors were associated with survival in patients with unresectable colorectal cancer, in addition to PTR, identified the following independent prognostic variables: primary tumor differentiation grade [4, 5, 11, 21,22,23, 25, 28, 29, 48], number of metastatic sites [2, 12, 14, 18, 21, 22, 25, 47, 66], primary tumor location [4, 6, 12, 22, 23, 25, 28, 66], extent of metastatic liver involvement [32, 48, 49, 75, 81, 89, 94], administration of chemotherapy [4, 14, 20, 24, 32, 39, 48, 49, 93], administration of anti-VEGF therapy [2, 13, 21, 23], CEA levels [5, 12,13,14, 28, 32], age [4, 6, 18, 22, 25, 28, 29], Eastern Cooperative Oncology Group performance status (ECOG-PS) [4, 14, 38,39,40], World Health Organization physiology score (WHO-PS) [2, 12, 49, 66], American Society of Anesthesiology (ASA) score [22, 93], primary tumor N-stage [2, 6, 18, 23, 25, 26], T-stage [25, 49], peritoneal dissemination [50, 83], adjacent organ invasion [20, 32], ascites [83], white blood cell count or neutrophil count [2, 4, 12], and levels of hemoglobin [2, 4], alkaline phosphatase [4, 66], aspartate aminotransferase [5], bilirubin [4], lactate dehydrogenase [38], and serum albumin [4]. The present review has quantified the duration of survival of each treatment strategy, quantified the survival benefit of PTR in different subgroups of patients, and has quantified the risk of morbidity of each individual treatment strategy, to hopefully assist the discussion within the multidisciplinary team on an individualized patient basis, as well as with the patient, to allow for an informed decision to be made.
References
de Haas RJ, Wicherts DA, Andreani P et al (2011) Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg 253:1069–1079
de Mestier L, Neuzillet C, Pozet A et al (2014) Is primary tumor resection associated with a longer survival in colon cancer and unresectable synchronous metastases? A 4-year multicentre experience. Eur J Surg Oncol 40:685–691
Kim CW, Baek JH, Choi GS et al (2016) The role of primary tumor resection in colorectal cancer patients with asymptomatic, synchronous unresectable metastasis: study protocol for a randomized controlled trial. Trials 17:34
Ahmed S, Leis A, Fields A et al (2014) Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: results from a large population-based cohort study. Cancer 120:683–691
Ahn HJ, Oh HS, Ahn Y et al (2014) Prognostic implications of primary tumor resection in stage IVB colorectal cancer in elderly patients. Ann Coloproctol 30:175–181
Aslam MI, Kelkar A, Sharpe D et al (2010) Ten years experience of managing the primary tumours in patients with stage IV colorectal cancers. Int J Surg 8:305–313
Beham A, Rentsch M, Pullmann K et al (2006) Survival benefit in patients after palliative resection vs non-resection colon cancer surgery. World J Gastroenterol 12:6634–6638
Chan TW, Brown C, Ho CC et al (2010) Primary tumor resection in patients presenting with metastatic colorectal cancer: analysis of a provincial population-based cohort. Am J Clin Oncol 33:52–55
Cook AD, Single R, McCahill LE (2005) Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 12:637–645
Costi R, Mazzeo A, Di Mauro D et al (2007) Palliative resection of colorectal cancer: does it prolong survival? Ann Surg Oncol 14:2567–2576
Crane CH, Janjan NA, Abbruzzese JL et al (2001) Effective pelvic symptom control using initial chemoradiation without colostomy in metastatic rectal cancer. Int J Radiat Oncol Biol Phys 49:107–116
Faron M, Pignon JP, Malka D et al (2015) Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur J Cancer 51:166–176
Ghiringhelli F, Bichard D, Limat S et al (2014) Bevacizumab efficacy in metastatic colorectal cancer is dependent on primary tumor resection. Ann Surg Oncol 21:1632–1640
Gresham G, Renouf DJ, Chan M et al (2014) Association between palliative resection of the primary tumor and overall survival in a population-based cohort of metastatic colorectal cancer patients. Ann Surg Oncol 21:3917–3923
Gulack BC, Nussbaum DP, Keenan JE et al (2016) Surgical resection of the primary tumor in stage IV colorectal cancer without metastasectomy is associated with improved overall survival compared with chemotherapy/radiation therapy alone. Dis Colon Rectum 59:299–305
He WZ, Rong YM, Jiang C et al (2016) Palliative primary tumor resection provides survival benefits for the patients with metastatic colorectal cancer and low circulating levels of dehydrogenase and carcinoembryonic antigen. Chin J Cancer 35:58
Ichikawa Y, Goto A, Kobayashi N et al (2013) Does resection of primary lesions show survival benefit for stage IV colorectal cancer patients with unresectable metastases? Hepatogastroenterology 60:1945–1949
Ishihara S, Hayama T, Yamada H et al (2014) Prognostic impact of primary tumor resection and lymph node dissection in stage IV colorectal cancer with unresectable metastasis: a propensity score analysis in a multicenter retrospective study. Ann Surg Oncol 21:2949–2955
Ishihara S, Nishikawa T, Tanaka T et al (2015) Benefit of primary tumor resection in stage IV colorectal cancer with unresectable metastasis: a multicenter retrospective study using a propensity score analysis. Int J Colorectal Dis 30:807–812
Jeong SJ, Yoon YS, Lee JB et al (2017) Palliative surgery for colorectal cancer with peritoneal metastasis: a propensity-score matching analysis. Surg Today 47:159–165
Karoui M, Roudot-Thoraval F, Mesli F et al (2011) Primary colectomy in patients with stage IV colon cancer and unresectable distant metastases improves overall survival: results of a multicentric study. Dis Colon Rectum 54:930–938
Kim SK, Lee CH, Lee MR et al (2012) Multivariate analysis of the survival rate for treatment modalities in incurable stage IV colorectal cancer. J Kor Soc Coloproctol 28:35–41
Samalavicius NE, Dulskas A, Baltruskeviciene E et al (2016) Asymptomatic primary tumour in incurable metastatic colorectal cancer: is there a role for surgical resection prior to systematic therapy or not? Wideochirurgia i inne techniki maloinwazyjne = Videosurgery and other miniinvasive techniques 11:274–282
Sigurdsson HK, Korner H, Dahl O et al (2008) Palliative surgery for rectal cancer in a national cohort. Colorectal Dis 10:336–343
t Lam-Boer J, Van der Geest LG, Verhoef C et al (2016) Palliative resection of the primary tumor is associated with improved overall survival in incurable stage IV colorectal cancer: a nationwide population-based propensity-score adjusted study in the Netherlands. Int J Cancer 139:2082–2094
Takada T, Tsutsumi S, Takahashi R et al (2016) Control of primary lesions using resection or radiotherapy can improve the prognosis of metastatic colorectal cancer patients. J Surg Oncol 114:75–79
Tanoue Y, Tanaka N, Nomura Y (2010) Primary site resection is superior for incurable metastatic colorectal cancer. World J Gastroenterol 16:3561–3566
Tarantino I, Warschkow R, Worni M et al (2015) Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: a population-based, propensity score-adjusted trend analysis. Ann Surg 262:112–120
Tsang WY, Ziogas A, Lin BS et al (2014) Role of primary tumor resection among chemotherapy-treated patients with synchronous stage IV colorectal cancer: a survival analysis. J Gastrointest Surg 18:592–598
Venderbosch S, de Wilt JH, Teerenstra S et al (2011) Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol 18:3252–3260
Wang Z, Liang L, Yu Y et al (2016) Primary tumour resection could improve the survival of unresectable metastatic colorectal cancer patients receiving bevacizumab-containing chemotherapy. Cell Physiol Biochem 39:1239–1246
Yoon YS, Kim CW, Lim SB et al (2014) Palliative surgery in patients with unresectable colorectal liver metastases: a propensity score matching analysis. J Surg Oncol 109:239–244
Al-Sanea N, Isbister WH (2004) Is palliative resection of the primary tumour, in the presence of advanced rectal cancer, a safe and useful technique for symptom control? ANZ J Surg 74:229–232
Cetin B, Kaplan MA, Berk V et al (2013) Bevacizumab-containing chemotherapy is safe in patients with unresectable metastatic colorectal cancer and a synchronous asymptomatic primary tumor. Jpn J Clin Oncol 43:28–32
Michel P, Roque I, Di Fiore F et al (2004) Colorectal cancer with non-resectable synchronous metastases: should the primary tumor be resected? Gastroenterol Clin Biol 28:434–437
Scoggins CR, Meszoely IM, Blanke CD et al (1999) Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol 6:651–657
Temple LK, Hsieh L, Wong WD et al (2004) Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol 22:3475–3484
Watanabe A, Yamazaki K, Kinugasa Y et al (2014) Influence of primary tumor resection on survival in asymptomatic patients with incurable stage IV colorectal cancer. Int J Clin Oncol 19:1037–1042
Wong SF, Wong HL, Field KM et al (2016) Primary tumor resection and overall survival in patients with metastatic colorectal cancer treated with palliative intent. Clin Colorectal Cancer 15:e125–e132
Yun JA, Huh JW, Park YA et al (2014) The role of palliative resection for asymptomatic primary tumor in patients with unresectable stage IV colorectal cancer. Dis Colon Rectum 57:1049–1058
Alawadi Z, Phatak UR, Hu CY et al (2017) Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer 123:1124–1133
Benoist S, Pautrat K, Mitry E et al (2005) Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. Br J Surg 92:1155–1160
Boselli C, Renzi C, Gemini A et al (2013) Surgery in asymptomatic patients with colorectal cancer and unresectable liver metastases: the authors’ experience. OncoTargets Ther 6:267–272
Evans MD, Escofet X, Karandikar SS et al (2009) Outcomes of resection and non-resection strategies in management of patients with advanced colorectal cancer. World J Surg Oncol 7:28
Matsumoto T, Hasegawa S, Matsumoto S et al (2014) Overcoming the challenges of primary tumor management in patients with metastatic colorectal cancer unresectable for cure and an asymptomatic primary tumor. Dis Colon Rectum 57:679–686
Miyamoto Y, Watanabe M, Sakamoto Y et al (2014) Evaluation of the necessity of primary tumor resection for synchronous metastatic colorectal cancer. Surg Today 44:2287–2292
Niitsu H, Hinoi T, Shimomura M et al (2015) Up-front systemic chemotherapy is a feasible option compared to primary tumor resection followed by chemotherapy for colorectal cancer with unresectable synchronous metastases. World J Surg Oncol 13:162
Seo GJ, Park JW, Yoo SB et al (2010) Intestinal complications after palliative treatment for asymptomatic patients with unresectable stage IV colorectal cancer. J Surg Oncol 102:94–99
Stelzner S, Hellmich G, Koch R et al (2005) Factors predicting survival in stage IV colorectal carcinoma patients after palliative treatment: a multivariate analysis. J Surg Oncol 89:211–217
Tebbutt NC, Norman AR, Cunningham D et al (2003) Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut 52:568–573
Moher D, Liberati A, Tetzlaff J et al (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341
Shea BJ, Reeves BC, Wells G et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Higgins JPT, Green S (eds) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from http://handbook.cochrane.org
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Wallace BC, Dahabreh IJ, Trikalinos TA et al (2012) Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 49. https://doi.org/10.18637/jss.v049.i05
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–2834
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (2014)
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, London
Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127:820–826
Chang HS, Lee KY, Lau JWL et al (2017) Modern day palliative chemotherapy for metastatic colorectal cancer (MCRC) in an Asian population. does colonic resection affect survival? and a retrospective cohort study. Gastroenterology 152(5 Supplement 1):S1258
Costi R, Di Mauro D, Giordano P et al (2010) Impact of palliative chemotherapy and surgery on management of stage IV incurable colorectal cancer. Ann Surg Oncol 17:432–440
Ferrand F, Malka D, Bourredjem A et al (2013) Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Federation Francophone de Cancerologie Digestive 9601. Eur J Cancer 49:90–97
Kodaz H, Erdogan B, Hacibekiroglu I et al (2015) Primary tumor resection offers higher survival advantage in KRAS mutant metastatic colorectal cancer patients. Hepatogastroenterology 62:876–879
Yun HR, Lee WY, Lee WS et al (2007) The prognostic factors of stage IV colorectal cancer and assessment of proper treatment according to the patient’s status. Int J Colorectal Dis 22:1301–1310
Ahmed S, Fields A, Pahwa P et al (2015) Surgical resection of primary tumor in asymptomatic or minimally symptomatic patients with stage IV colorectal cancer: a canadian province experience. Clin Colorectal Cancer 14:e41–e47
Bacon HE, Martin PV (1964) The rationale of palliative resection for primary cancer of the colon and rectum complicated by liver and lung metastasis. Dis Colon Rectum 7:211–217
Bajwa A, Blunt N, Vyas S et al (2009) Primary tumour resection and survival in the palliative management of metastatic colorectal cancer. Eur J Surg Oncol 35:164–167
Cummins ER, Vick KD, Poole GV (2004) Incurable colorectal carcinoma: the role of surgical palliation. Am Surg 70:433–437
Duraker N, Civelek Caynak Z, Hot S (2014) The impact of primary tumor resection on overall survival in patients with colorectal carcinoma and unresectable distant metastases: a prospective cohort study. Int J Surg 12:737–741
Frago R, Kreisler E, Biondo S et al (2010) Outcomes in the management of obstructive unresectable stage IV colorectal cancer. Eur J Surg Oncol 36:1187–1194
Galizia G, Lieto E, Orditura M et al (2008) First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg 143:352–358 (discussion 358)
Hatano S, Matsuzawa T, Kumamoto K et al (2013) Efficacy of oxaliplatin-based chemotherapy in Stage IV colorectal cancer patients with peritoneal carcinomatosis. Gan to kagaku ryoho Cancer & chemotherapy 40:1981–1983
Johnson WR, McDermott FT, Pihl E et al (1981) Palliative operative management in rectal carcinoma. Dis Colon Rectum 24:606–609
Kaufman MS, Radhakrishnan N, Roy R et al (2008) Influence of palliative surgical resection on overall survival in patients with advanced colorectal cancer: a retrospective single institutional study. Colorectal Dis 10:498–502
Klaver YL, Lemmens VE, de Hingh IH (2013) Outcome of surgery for colorectal cancer in the presence of peritoneal carcinomatosis. Eur J Surg Oncol 39:734–741
Kodaz H, Erdogan B, Hacibekiroglu I et al (2015) Impact of bevacizumab on survival outcomes in primary tumor resected metastatic colorectal cancer. Med Oncol 32:441
Konyalian VR, Rosing DK, Haukoos JS et al (2007) The role of primary tumour resection in patients with stage IV colorectal cancer. Colorectal Dis 9:430–437
Lau JWL, Chang HS, Lee KY et al (2017) Primary tumour resection confers benefit on the overall survival of patients with metastatic colorectal cancer (MCRC) and unresectable metastases. Gastroenterology 152(5 Supplement 1):S1302
Law WL, Chan WF, Lee YM et al (2004) Non-curative surgery for colorectal cancer: critical appraisal of outcomes. Int J Colorectal Dis 19:197–202
Liu SK, Church JM, Lavery IC et al (1997) Operation in patients with incurable colon cancer–is it worthwhile? Dis Colon Rectum 40:11–14
Longo WE, Ballantyne GH, Bilchik AJ et al (1988) Advanced rectal cancer. What is the best palliation? Dis Colon Rectum 31:842–847
Mahteme H, Pahlman L, Glimelius B et al (1996) Prognosis after surgery in patients with incurable rectal cancer: a population-based study. Br J Surg 83:1116–1120
Makela J, Haukipuro K, Laitinen S et al (1990) Palliative operations for colorectal cancer. Dis Colon Rectum 33:846–850
Maroney S, de Paz CC, Reeves ME et al (2018) Benefit of surgical resection of the primary tumor in patients undergoing chemotherapy for stage IV colorectal cancer with unresected metastasis. J Gastrointest Surg 22:460–466
Mik M, Dziki L, Galbfach P et al (2010) Resection of the primary tumour or other palliative procedures in incurable stage IV colorectal cancer patients? Colorectal Dis 12:e61–e67
Modlin J, Walker SJ (1949) Palliative resections in cancer of the colon and rectum. Cancer 2:767–776
Moran MR, Rothenberger DA, Lahr CJ et al (1987) Palliation for rectal cancer. Resection? Anastomosis? Arch Surg 122:640–643
Okuyama K, Onoda S, Tohnosu N et al (1988) The prognostic significance of resection of primary tumor in gastric and colorectal cancer patients with synchronous liver metastasis. Jpn J Surg 18:7–17
Platell C, Ng S, O’Bichere A et al (2011) Changing management and survival in patients with stage IV colorectal cancer. Dis Colon Rectum 54:214–219
Ruo L, Gougoutas C, Paty PB et al (2003) Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg 196:722–728
Stearns MW Jr, Binkley GE (1954) Palliative surgery for cancer of the rectum and colon. Cancer 7:1016–1019
van Rooijen KL, Shi Q, Goey KKH et al (2018) Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: individual patient data analysis of first-line randomised trials from the ARCAD database. Eur J Cancer 91:99–106
Vatandoust S, Roy AC, Price TJ et al (2015) Survival impact of primary tumor resection in patients (pts) with unresectable metastatic colorectal cancer (mCRC): findings from the South Australian Metastatic Colorectal Cancer Registry (SAMCRC). J Clin Oncol 33:e14675
Verberne CJ, de Bock GH, Pijl MEJ et al (2011) Palliative resection of the primary tumour in stage IV rectal cancer. Colorectal Dis 14:314–319
Xu H, Xia Z, Jia X et al (2015) Primary tumor resection is associated with improved survival in stage IV colorectal cancer: an instrumental variable analysis. Sci Rep 5:16516
Zhang RX, Ma WJ, Gu YT et al (2017) Primary tumor location as a predictor of the benefit of palliative resection for colorectal cancer with unresectable metastasis. World J Surg Oncol 15:138
Biondo S, Frago R, Kreisler E et al (2017) Impact of resection versus no resection of the primary tumor on survival in patients with colorectal cancer and synchronous unresectable metastases: protocol for a randomized multicenter study (CR101). Int J Colorectal Dis 32:1085–1090
Cotte E, Villeneuve L, Passot G et al (2015) GRECCAR 8: impact on survival of the primary tumor resection in rectal cancer with unresectable synchronous metastasis: a randomized multicentre study. BMC Cancer 15:47
Lam-Boer J, Mol L, Verhoef C et al (2014) The CAIRO4 study: the role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer—a randomized phase III study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer 14:1–7
Rahbari NN, Lordick F, Fink C et al (2012) Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS–a randomised controlled multicentre trial (ISRCTN30964555). BMC Cancer 12:142
Anwar S, Peter MB, Dent J et al (2012) Palliative excisional surgery for primary colorectal cancer in patients with incurable metastatic disease. Is there a survival benefit? A systematic review. Colorectal Dis 14:920–930
Clancy C, Burke JP, Barry M et al (2014) A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol 21:3900–3908
Verhoef C, de Wilt JH, Burger JWA et al (2011) Surgery of the primary in stage IV colorectal cancer with unresectable metastases. Eur J Cancer 47:S61–S66
Sarela AI, Guthrie JA, Seymour MT et al (2001) Non-operative management of the primary tumour in patients with incurable stage IV colorectal cancer. Br J Surg 88:1352–1356
Walker E, Hernandez AV, Kattan MW (2008) Meta-analysis: its strengths and limitations. Clevel Clin J Med 75:431–439
Goldberg RM, Sargent DJ, Morton RF et al (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30
Golfinopoulos V, Salanti G, Pavlidis N et al (2007) Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: a meta-analysis. Lancet Oncol 8:898–911
Hochster HS, Hart LL, Ramanathan RK et al (2008) Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 26:3523–3529
Tournigand C, Andre T, Achille E et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Emmanouilides C, Sfakiotaki G, Androulakis N et al (2007) Front-line bevacizumab in combination with oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX) in patients with metastatic colorectal cancer: a multicenter phase II study. BMC Cancer 7:91
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Van Cutsem E, Kohne CH, Hitre E et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Van Cutsem E, Kohne CH, Lang I et al (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29:2011–2019
Van Cutsem E, Rivera F, Berry S et al (2009) Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 20:1842–1847
Kleespies A, Fuessl KE, Seeliger H et al (2009) Determinants of morbidity and survival after elective non-curative resection of stage IV colon and rectal cancer. Int J Colorectal Dis 24:1097–1109
Watt DG, Horgan PG, McMillan DC (2015) Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery 157:362–380
Stillwell AP, Buettner PG, Siu SK et al (2011) Predictors of postoperative mortality, morbidity, and long-term survival after palliative resection in patients with colorectal cancer. Dis Colon Rectum 54:535–544
Flanigan RC, Salmon SE, Blumenstein BA et al (2001) Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 345:1655–1659
Mickisch GH, Garin A, van Poppel H et al (2001) Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 358:966–970
van der Burg ME, Vergote I, Gynecological Cancer Group of the E (2003) The role of interval debulking surgery in ovarian cancer. Curr Oncol Rep 5:473–481
Holmgren L, O’Reilly MS, Folkman J (1995) Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1:149–153
Qian CN, Berghuis B, Tsarfaty G et al (2006) Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Can Res 66:10365–10376
van der Wal GE, Gouw AS, Kamps JA et al (2012) Angiogenesis in synchronous and metachronous colorectal liver metastases: the liver as a permissive soil. Ann Surg 255:86–94
Holzel D, Eckel R, Emeny RT et al (2010) Distant metastases do not metastasize. Cancer Metastasis Rev 29:737–750
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Risk of bias graph. Review authors’ judgments about each risk of bias item presented as percentages across all included studies. (TIFF 20 kb)
Supplementary Figure 2
Risk of bias summary. Review authors’ judgments about each risk of bias item for each included study. Footnotes: green plus sign = low risk of bias, yellow question mark = unclear risk of bias, red minus sign = high risk of bias. (TIFF 100 kb)
Supplementary Figure 3
Forest plot comparing overall survival between PTR and PTI as a time-to-event outcome. Footnotes: PTR, primary tumor resection; PTI, primary tumor intact; CI, confidence interval; SD, standard deviations; HR, hazard ratio, an HR of less than one favored the PTR group; IV, inverse variance; Random, random-effects model; Test for overall effect: Z statistic with P value; Test for heterogeneity: chi2 statistic with its degrees of freedom (df) and P value; Inconsistency among results: I2. (TIFF 65 kb)
Supplementary Figure 4
Forest plot comparing duration of overall survival between PTR and PTI as a continuous variable in months. Footnotes: PTR, primary tumor resection; PTI, primary tumor intact; CI confidence interval; MD, mean difference, an MD higher than zero favored the PTR group; IV, inverse variance; Random, random-effects model; Test for overall effect: Z statistic with P value; Test for heterogeneity: chi2 statistic with its degrees of freedom (df) and P value; Inconsistency among results: I2. (TIFF 66 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Simillis, C., Kalakouti, E., Afxentiou, T. et al. Primary Tumor Resection in Patients with Incurable Localized or Metastatic Colorectal Cancer: A Systematic Review and Meta-analysis. World J Surg 43, 1829–1840 (2019). https://doi.org/10.1007/s00268-019-04984-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-04984-2