Abstract
Background
Necrotizing soft tissue infections (NSTI) are emergency surgical conditions with severe physiologic and metabolic derangement. These infections are associated with increased rates of mortality and morbidity worldwide, particularly in developing countries if not diagnosed and treated early.
Methods
This prospective, observational cohort study includes all patients aged 12 and above who presented at Department of Surgery, University Teaching Hospital of Kigali from April 2016 to January 2017 with NSTI. We describe epidemiology, operative management, and outcomes of care. We determined risk factors for mortality using multivariate logistic regression.
Results
We identified 175 patients with confirmed diagnosis of NSTI. The majority of patients (53%) were male, and the mean age was 44 years. The median duration of symptoms was 8 days [interquartile range (IQR) 5–14]. The median length of hospital stay was 23 days (IQR 8–41). The overall mortality was 26%. Multivariate regression analysis revealed four independent predictors of mortality: presence of shock at admission [odds ratio (OR) 14.15, 95% confidence interval (CI) 0.96–208.01, p = 0.050], renal failure (OR 8.92, 95% CI 1.55–51.29, p = 0.014), infection located on the trunk (OR 5.60, 95% CI 0.99–31.62, p = 0.050), and presence of skin gangrene (OR 4.04, 95% CI 1.18–13.76, p = 0.026).
Conclusion
In Rwanda, NSTI mortality is high and associated with advanced disease. It is imperative that efforts are focused on early consultation, diagnosis, and surgical management to prevent adverse outcomes.
Similar content being viewed by others
Introduction
Necrotizing soft tissue infections (NSTIs) are severe infections of any layer of soft tissue compartment including superficial and deep soft tissues [1, 2]. NSTIs were first recognized and described by Hippocrates in 500 B.C. as a complication of erysipelas [3]. In 1871, Jones reported 2642 cases of what he termed “hospital gangrene” with a mortality rate of 46% [4]. NSTIs are commonly polymicrobial infections characterized by a rapid spread, clinical deterioration, and increased mortality and morbidity [5,6,7]. For many patients, there is a history of trauma or surgery with wound contamination. NSTIs can occur in any area of the body with extremities being the most commonly involved part [2].
The incidence of this condition varies from site to site with national and regional variation in both etiology and microbiology [8].The worldwide incidence of NSTIs is estimated at 0.4 cases per 100,000. In USA, there were more than 13,000 cases in 2007, with an annual incidence of 4.5 per 100,000 population [9]. In the South Pacific, the incidence is estimated at 6.1 per 100,000 population [7]. In Africa and Asia, no true incidence is known, but the estimate is more than 1 case per 100,000 population per year [5, 9,10,11]. Mortality in patients with NSTIs is high, ranging from 14 to 42% [6, 10, 12,13,14].
Early diagnosis and treatment are essential for survival. Treatment consists of broad-spectrum antibiotics, wide surgical debridement, and supportive care [2, 8, 15]. Antibiotic treatment is initially broad spectrum and then tailored to antimicrobial susceptibilities of isolated organisms [2, 16, 17]. Patients may require multiple debridements or amputations to ensure adequate source control [6, 12].
Rwanda is a densely populated, low-income country in East Africa [18]. University Teaching Hospital of Kigali (Centre Hopitalier Universitaire de Kigali, CHUK) is a referral hospital in Kigali, Rwanda, that serves as referral hospital for 44 district hospitals and over 6 million people [19]. The hospital has a capacity of 513 beds with a 7-bed intensive care unit (ICU). The department of surgery accounts for 146 hospital beds and 6 main operating rooms. Emergency surgical conditions account for 70% of general surgery operations with soft tissue infections accounting for a significant proportion of these cases [20]. No data are available in Rwanda on the clinical characteristics and outcomes of care for patients with NSTI. The aim of this study was to describe the epidemiology, management, and outcomes of patients with NSTI at tertiary referral hospital in Rwanda and determine admission factors associated with mortality.
Methods
This was a prospective study of all patients aged 12 and above with a diagnosis of NSTI managed by the Department of Surgery at CHUK over a 10-month period (April 2016–January 2017). NSTI was defined as any patient with severe skin and soft tissue infection confirmed by the treating surgeon intra-operatively.
Data were collected on demographics, comorbidities, clinical presentation, precipitating event, operative management, complications, and duration of hospital stay. Cardiac disease included hypertension and congestive heart disease. Renal disease included both chronic and acute renal failure. This included patients with a prior history of renal disease or those who had creatinine of >88.4 µmol/L on admission. Pulmonary disease included patients with asthma, chronic obstructive pulmonary disease, and history of tuberculosis. We defined shock as any clinical status of persistent hypotension (systolic blood pressure less than 90 mmHg) despite adequate intravenous fluid resuscitation. Tachycardia was defined as a heart rate greater than 110 beats per minute. Leukocytosis was defined as a white blood cell counts greater than 10,000 µmol/L. Anemia was defined as hemoglobin less than 10 g/dL. Complications included pneumonia, unplanned intubation, prolonged ventilator duration, cardiac arrhythmia, cardiopulmonary arrest, acute kidney injury, surgical site infection, septic shock, and death. All outcomes were measured in-hospital. Patients were followed until death or hospital discharge.
NSTI was classified as superficial necrotizing infection (any soft tissue infection not reaching the fascia), necrotizing fasciitis (deep infection involving the fascia), pyomyositis, Fournier’s gangrene (necrotizing infection of the perineum and genitalia), and wet gangrene of extremities based on intra-operative findings [17].
Data were collected using a pretested questionnaire during recruitment and follow-up of the patients. A database was created using Excel, and STATA 13.0 software was used for statistical analysis. We used frequencies and percentages for categorical data, and median and interquartile range (IQR) for continuous data. The association between variables was assessed using Chi-square test. The relationship between risk factors and mortality was studied using a multivariate logistic regression analysis. Variables with a p value <0.1 on bivariate analysis were incorporated in a multivariate logistic model analysis. A p value <0.05 on multivariate analysis was considered statistically significant.
The institutional review boards of the University of Rwanda College of Medicine and Health Sciences and CHUK Ethics Committee approved this study.
Results
Over a 10-month period (April 2016–January 2017), 198 patients at CHUK had suspected NSTI, with 175 diagnoses confirmed by intra-operative assessment (Fig. 1). Ninety-two (52.6%) patients were male and 83 (47.4%) patients were female (Table 1). The mean age was 43.8 years (range 12 and 92 years). The majority of patients were from Kigali City (43%) and Eastern Province (32%). Eighty-six (49%) patients presented with comorbidities with the most common being cardiac disease (n = 29, 17%), diabetes mellitus (n = 28, 16%), smoking (n = 23, 13%), and human immunodeficiency virus (HIV) infection (n = 20, 11%).
Eighty-nine patients (51%) had a precipitating event with the most common being postoperative infection in 43 (25%) patients and trauma in 37 (21%) patients. Eighty-six patients (49%) had no precipitating event (Table 2). The most common presenting symptoms included pus discharge (n = 153, 87%), edema (n = 149, 85%), pain (n = 119, 68%), and skin necrosis (n = 118, 67%). The median length of symptoms was 8 days (IQR 5–14).
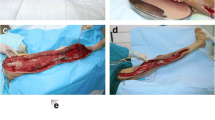
The most common types of NSTI were necrotizing fasciitis (n = 74, 42%) followed by gangrene of extremities (n = 50, 29%), Fournier’s gangrene (n = 25, 14%), superficial necrotizing infections (n = 16, 9%), and pyomyositis (n = 10, 6%). The most commonly involved tissue planes were the deep fascia (n = 154, 88%) and subcutaneous tissues (n = 139, 79%). The most commonly involved body parts were lower extremities (n = 94, 54%) and trunk (n = 44, 25%).
The time patients spent in hospital from the presumptive diagnosis to operation varied between 30 min and 240 h with a median of 18 h. We defined delayed operative time as time from admission to surgery more than 12 h, and there was no difference in patients taken to surgery within 12 h or after (p = 0.11). All patients (N = 175) underwent operation, with the most common initial operations debridement (n = 90, 51%) and amputation or disarticulation (n = 52, 30%). A second operation was performed in 24 patients with the most common second operations being skin graft (n = 12, 50%) and amputation or disarticulation (n = 5, 21%) (Table 3). In total, 57 (33%) patients underwent amputation or disarticulation.
Postoperative complications were noted in 89 patients (51%). The most common complications were septic shock (n = 50, 29%) and pneumonia (n = 29, 17%). Eight patients (5%) were admitted in ICU with a median ICU duration of 3 days (IQR 1.5–4). The overall mortality was 46 (26%). The median length of hospital stay for survivors was 29 days (IQR 15–48).
Factors associated with mortality on multivariate analysis included: presence of shock at admission [odds ratio (OR) 14.15, 95% confidence interval (CI) 0.96–208.01, p = 0.050], renal failure (OR 8.92, 95% CI 1.55–51.29, p = 0.014), infection located to the trunk (OR 5.60, 95% CI 0.99–31.62, p = 0.050), and presence of skin gangrene (OR 4.04, 95% CI 1.18–13.76, p = 0.026) (Table 4).
Discussion
Overall, the epidemiology and clinical presentation of NSTIs in this study were similar to other studies [6, 12]. This study found a lower mean age, which may be associated with an overall lower mean population age in Rwanda compared with high-income countries [21]. Only 6.7% of Rwandan population is 55 years or older [18, 21]. Most patients were referred from Kigali or the Eastern Province, which is similar to the overall surgical referral pattern for this hospital [20]. The incidence of comorbidities was lower in this study compared with other studies, which may be related to the lower patient age [12]. The most common presentation was pus discharge, edema beyond erythema, pain, focal skin gangrene, and skin blistering, consistent with clinical presentation described elsewhere [1, 13]. Postoperative infection and trauma as precipitating event were similar to other studies [5, 13]. Lower extremities were the most affected body part, with a high incidence of truncal involvement which may be associated with the high incidence of Fournier’s in this study [5, 13, 22].
The mean symptom duration prior to presentation was 8 days. There are many potential reasons for this long symptom duration, which were not assessed in this study. There may be a delay in patient recognition, delay in health care presentation, or transfer delays. In addition, patients with shorter duration of symptoms may have had more aggressive disease and potentially died prior to transfer to the referral hospital. Furthermore, the fact that patients were delayed (average symptom duration of 8 days) could be linked to nonsignificant difference in mortality in regards to when patient was taken to theater. However, some authors reported significant difference between mortality and time to intervention from symptom onset or hospital admission [23]. More studies related to health-seeking behavior and pre-hospital challenges in systems of care would help to better understand and determine modifiable factors to improve the mortality and morbidity.
Most patients underwent a single debridement, which is lower than other studies [6, 12]. There are two potential reasons for the lower debridement rate. One possibility is that the decreased number of second relook is due to surgical delivery system issues such as lack of operating room time. Another possibility is that patients had their infection controlled with the initial operation and did not need re-debridement. The amputation rate was 33%, which is higher than other studies [6, 12]. Other studies have noted an association limb amputation and delayed presentation [7, 15, 24]. Amputation may be viewed as an efficient form of source control in this setting. In addition, flap reconstruction is more challenging in this environment limiting the limb salvage rate.
The overall mortality in our study was 26% similar to other studies [2, 6, 9, 22, 25, 26]. However, some centers have lower mortality rates [11, 13]. As suggested in other studies, differences in mortality may be due to differences in practice patterns, microbiology, or epidemiology [6, 12]. Delayed operative management has been associated with increased mortality rate [7, 10, 13]. In our study, similar to Kao et al. [6], delayed operative management was not associated with increased mortality. In contrast to other studies, factors like advanced age, immunosuppression by human immunodeficiency virus infection, and elevated white blood count were not significantly associated with mortality in our study [13, 22, 26, 27]. This is likely due to different population demographics with a lower mean age and lower incidence of comorbidities.
Multiple regression analysis revealed four independent predictors of mortality: shock on admission, renal failure, truncal involvement, and skin gangrene. Many of these predicting factors correlate with other published studies [8, 26, 28]. Shock and renal failure indicate patients with critical illness and organ failure. Due to resource limitations, other measures of organ failure are more difficult to assess. This hospital does not routinely have access to laboratory studies such as bilirubin, coagulation factors, lactate, or arterial blood gas. Therefore, the incidence of organ failure is likely underestimated in this patient population.
Patients with NSTI often have severe physiologic and metabolic derangements that predispose the patient to develop organ failure, with many patients requiring ICU admission [1]. In our study, only 5% of patients were admitted in ICU for aggressive resuscitation and supportive care. This low rate of ICU admission may be due to several factors. One reason may be due to the shortage of ICU space (only 7 ICU beds shared by the entire hospital), which could be improved by increasing the infrastructure and equipment to accommodate patients at an early stage of postoperative management. Another possibility for the low ICU admission rate may be that patients with septic shock on admission died prior to ICU admission.
There are limitations to be considered while interpreting the results. These data are from a single hospital in Rwanda which functions as a tertiary referral hospital for 44 district hospitals. The hospital catchment area represents 60% of the Rwandan population. However, there are likely patients that die before reaching this tertiary level hospital. There were no data collected on management prior to hospital admission, and we did not collect data on illness severity scores used in other studies. The degree of source control at time of operation was not assessed limiting some analysis. All outcomes were in-hospital, and we did not collect data on long-term functional outcomes. The long-term complications would help to characterize the disease sequelae and identify the factors related to favorable and unfavorable NSTI long-term functional outcome.
Conclusion
Our study showed that patients with NSTI in Rwanda have a high morbidity and mortality, and most admission predictors of mortality can be prevented by the adequate understanding and knowledge of this condition for a proper diagnosis, management, and timely transfer.
References
Anaya DA, Bulger EM, Kwon YS, Kao LS, Evans H, Nathens AB (2009) Predicting death in necrotizing soft tissue infections: a clinical score. Surg Infect (Larchmt) 10(6):517–522
Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P, Machairas A (2014) Current concepts in the management of necrotizing fasciitis. Front Surg 1:36
Descamps V, Aitken J, Lee MG (1994) Hippocrates on necrotising fasciitis. Lancet 344(8921):556
Jones J (1871) Surgical memoirs of the war of the rebellion. In: US Commission (ed) Investigation upon the nature, causes and treatment of hospital gangrene as prevailed in the confederate armies 1861–1865. Riverside Press, New York
Hua C, Sbidian E, Hemery F, Decousser JW, Bosc R, Amathieu R et al (2015) Prognostic factors in necrotizing soft-tissue infections (NSTI): a cohort study. J Am Acad Dermatol 73(6):1006-12.e8
Kao LS, Lew DF, Arab SN, Todd SR, Awad SS, Carrick MM et al (2011) Local variations in the epidemiology, microbiology, and outcome of necrotizing soft-tissue infections: a multicenter study. Am J Surg 202(2):139–145
Kha P, Colot J, Gervolino S, Guerrier G (2017) Necrotizing soft-tissue infections in New Caledonia: epidemiology, clinical presentation, microbiology, and prognostic factors. Asian J Surg 40(4):290–294
Hakkarainen TW, Kopari NM, Pham TN, Evans HL (2014) Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg 51(8):344–362
Soltani AM, Best MJ, Francis CS, Allan BJ, Askari M, Panthaki ZJ (2014) Trends in the incidence and treatment of necrotizing soft tissue infections: an analysis of the National Hospital Discharge Survey. J Burn Care Res 35(5):449–454
Magala J, Makobore P, Makumbi T, Kaggwa S, Kalanzi E, Galukande M (2014) The clinical presentation and early outcomes of necrotizing fasciitis in a Ugandan Tertiary Hospital—a prospective study. BMC Res Notes 7:476
Faro S, Faro JP (2012) Necrotizing soft-tissue infections in obstetric and gynecologic patients. Clin Obstet Gynecol 55(4):875–887
Faraklas I, Yang D, Eggerstedt M, Zhai Y, Liebel P, Graves G et al (2016) A multi-center review of care patterns and outcomes in necrotizing soft tissue infections. Surg Infect (Larchmt) 17(6):773–778
Kalaivani V, Hiremath BV, Indumathi VA (2013) Necrotising soft tissue infection-risk factors for mortality. J Clin Diagn Res 7(8):1662–1665
Yahav D, Duskin-Bitan H, Eliakim-Raz N, Ben-Zvi H, Shaked H, Goldberg E et al (2014) Monomicrobial necrotizing fasciitis in a single center: the emergence of Gram-negative bacteria as a common pathogen. Int J Infect Dis 28:13–16
Mishra SP, Singh S, Gupta SK (2013) Necrotizing soft tissue infections: surgeon’s prospective. Int J Inflam 2013:609628
Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL et al (2014) Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59(2):e10–e52
Sartelli M, Malangoni MA, May AK, Viale P, Kao LS, Catena F et al (2014) World Society of Emergency Surgery (WSES) guidelines for management of skin and soft tissue infections. World J Emerg Surg 9(1):57
Population of Rwanda: National Institute of Statistics of Rwanda. http://rwanda.opendataforafrica.org/mlhmqxf/rwanda-census
Petroze RT, Nzayisenga A, Rusanganwa V, Ntakiyiruta G, Calland JF (2012) Comprehensive national analysis of emergency and essential surgical capacity in Rwanda. Br J Surg 99(3):436–443
Rickard JL, Ntakiyiruta G, Chu KM (2015) Identifying gaps in the surgical training curriculum in Rwanda through evaluation of operative activity at a teaching hospital. J Surg Educ 72(4):e73–e81
Rwanda Age Structure: Index Mundi (2017). http://www.indexmundi.com/rwanda/age_structure.html
Martinschek A, Evers B, Lampl L, Gerngross H, Schmidt R, Sparwasser C (2012) Prognostic aspects, survival rate, and predisposing risk factors in patients with Fournier’s gangrene and necrotizing soft tissue infections: evaluation of clinical outcome of 55 patients. Urol Int 89(2):173–179
Bandyopadhyay D, Jacobs JV, Panchabhai TS (2016) What’s new in emergencies, trauma and shock? The tortuous path in the management of necrotizing fasciitis: is early surgical intervention critical? J Emerg Trauma Shock 9(1):22–27
Paramythiotis D, Koukoutsis H, Harlaftis N (2007) Necrotizing soft tissue infections. Surg Pract 11(1):17–28
Schuster L, Nunez DE (2012) Using clinical pathways to aid in the diagnosis of necrotizing soft tissue infections synthesis of evidence. Worldviews Evid Based Nurs 9(2):88–99
Obimakinde OS, Okoje VN, Akinmoladun VI, Fasola AO, Arotiba JT (2012) Retrospective evaluation of necrotizing fasciitis in University College Hospital, Ibadan. Niger J Clin Pract 15(3):344–348
Yaghoubian A, de Virgilio C, Dauphine C, Lewis RJ, Lin M (2017) Use of admission serum lactate and sodium levels to predict mortality in necrotizing soft-tissue infections. Arch Surg 142(9):840–846
Krieg A, Dizdar L, Verde PE, Knoefel WT (2014) Predictors of mortality for necrotizing soft-tissue infections: a retrospective analysis of 64 cases. Langenbecks Arch Surg 399(3):333–341
Acknowledgements
We acknowledge the contributions of residents in surgery rotating at CHUK during the study period, laboratory technicians, emergency, theater, and surgical ward nurses for data collection.
Author information
Authors and Affiliations
Contributions
CM led study design, protocol development, literature search, led data collection and analysis with support from JR, abstract and manuscript development, interpretation and dissemination of results. JR, CF, and FN supported study design, protocol development, literature search, results interpretation, and dissemination of results. All authors critically reviewed the manuscript and approved the final version for publication.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Mpirimbanyi, C., Rickard, J., Furaha, C. et al. Necrotizing Soft Tissue Infections at a Tertiary Referral Hospital in Rwanda: Epidemiology and Risk Factors for Mortality. World J Surg 42, 2314–2320 (2018). https://doi.org/10.1007/s00268-018-4515-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4515-z