Abstract
Background
In surgery, rapid hemostasis can be required in various settings and bleeding intensities to minimize complications related to blood loss. While effective hemostats are available for mild-to-moderate surgical bleeding, few are effective against challenging severe hemorrhage. We report the effectiveness and safety of the fibrin pad (FP), a novel combination hemostat (device/human biologic), in controlling severe soft-tissue bleeding as compared to the standard of care (SoC).
Methods
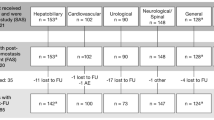
This randomized, controlled, superiority study enrolled subjects ≥18 years, requiring elective abdominal, retroperitoneal, pelvic, or thoracic (non-cardiac) surgery. A severe target bleeding site (TBS) was identified intra-operatively following which, subjects were randomized to the FP or the SoC group. Hemostatic status was observed at 4 min (primary endpoint) and 10 min post-randomization. Safety variables included TBS-related bleeding and thrombotic events.
Results
At 4 min post-randomization, 50/59 (84.7 %) subjects in the FP group and 16/32 (50 %) subjects in the SoC group achieved hemostasis without needing re-treatment (P < 0.0001). Compared to the SoC group, the FP group showed better hemostasis at 10 min post-randomization [58/59 (98.3 %) vs. 28/32 (87.5 %); P = 0.01], lower mean time to hemostasis (6.1 ± 13.5 vs. 17.8 ± 32.0 min), and a less frequent need for re-treatment (5.1 vs. 53.1 %). The triangular test for binary response demonstrated the FP to be superior to SoC (95 % CI 1.474–3.290; P < 0.0001). Safety profiles in both groups were similar to those typically observed after long-duration surgery.
Conclusion
The FP is safe and superior to SoC for controlling challenging severe soft-tissue bleeding encountered during intra-abdominal and thoracic surgical procedures.

Similar content being viewed by others
References
Stokes ME, Ye X, Shah M, Mercaldi K et al (2011) Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res 11:135
Marietta M, Facchini L, Pedrazzi P, Busani S, Torelli G (2006) Pathophysiology of bleeding in surgery. Transpl Proc 38(3):812–814
Tomizawa Y (2005) Clinical benefits and risk analysis of topical hemostats: a review. J Artif Organs 8(3):137–142
Gabay M (2006) Absorbable hemostatic agents. Am J Health Syst Pharm 63(13):1244–1253
Voils S (2007) Pharmacologic interventions for the management of critical bleeding. Pharmacotherapy 27(9 Pt 2):69S–84S
Ding H, Yuan JQ, Zhou JH, Zheng XY et al (2013) Systematic review and meta-analysis of application of fibrin sealant after liver resection. Curr Med Res Opin 29(4):387–394
Chapman WC, Wren SM, Lebovic GS, Malawer M et al (2002) Effective management of bleeding during tumor resection with a collagen-based hemostatic agent. Am Surg 68(9):802–807
Fischer CP, Wood CG, Shen J, Batiller J et al (2011) A randomized trial of aprotinin-free fibrin sealant versus absorbable hemostat. Clin Appl Thromb Hemost 17(6):572–577
Chan DY, Marshall FF (1999) Partial nephrectomy for centrally located tumors. Urology 54(6):1088–1091 (Discussion 1091–1082)
Richter F, Schnorr D, Deger S, Trk I et al (2003) Improvement of hemostasis in open and laparoscopically performed partial nephrectomy using a gelatin matrix-thrombin tissue sealant (FloSeal). Urology 61(1):73–77
Baumann P, Schumacher H, Husing J, Luntz S, Knaebel HP (2009) A randomized, controlled, prospective trial to evaluate the haemostatic effect of Lyostypt versus Surgical in arterial bypass anastomosis: “COBBANA” trial. Trials 10:91
Hollaus P, Pridun N (1994) The use of Tachocomb in thoracic surgery. J Cardiovasc Surg (Torino) 35(6 Suppl 1):169–170
Delgado AV, Kheirabadi BS, Fruchterman TM, Scherer M et al (2008) A novel biologic hemostatic dressing (fibrin patch) reduces blood loss and resuscitation volume and improves survival in hypothermic, coagulopathic Swine with grade V liver injury. J Trauma 64(1):75–80
Ward KR, Tiba MH, Holbert WH, Blocher CR et al (2007) Comparison of a new hemostatic agent to current combat hemostatic agents in a Swine model of lethal extremity arterial hemorrhage. J Trauma 63(2):276–283 (Discussion 283–274)
Fischer CP, Bochicchio G, Shen J, Patel B et al (2013) A prospective, randomized, controlled trial of the efficacy and safety of fibrin pad as an adjunct to control soft tissue bleeding during abdominal, retroperitoneal, pelvic, and thoracic surgery. J Am Coll Surg 217(3):385–393
Koea JB, Batiller J, Patel B, Shen J et al (2013) A phase III, randomized, controlled, superiority trial evaluating the fibrin pad versus standard of care in controlling parenchymal bleeding during elective hepatic surgery. HPB 15(1):61–70
Nativ O, Patel B, Shen J, Batiller J et al (2012) Safety and hemostatic efficacy of fibrin pad in partial nephrectomy: results of an open-label phase I and a randomized, standard-of-care-controlled phase I/II study. BMC Nephrol 13:147
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340:c332
Harmonized Tripartite Guideline for Good Clinical Practice (2012). http://www.ich.org. Accessed 27 July 2012
US Food and Drug Administration Regulations (2012). http://www.fda.gov/ScienceResearch/SpecialTopics/RunningClinicalTrials/. Accessed 27 July 2012
Declaration of Helsinki (2012). http://www.wma.net/en/30publications/10policies/b3/. Accessed 26 July 2012
European Union Trial Directive (2012). http://ec.europa.eu/health/human-use/clinical-trials/index. Accessed 26 July 2012
Guideline on the Clinical Investigation of Plasma Derived Fibrin Sealant/Haemostatic Products (CPMP/BPWG/1089/00). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003536.pdf
Acknowledgments
Fibrin pads were provided by Omrix Biopharmaceuticals, Ltd., Kiryat Ono, Israel. The authors retained full control of the manuscript content. The authors acknowledge the contribution of the following clinical development staff: Val Jarvis-Evans, Anna Lam, Gerry Leighton, Nicolas Aguirre, Cristina Dyogi, David Shah, and Linda Lin. The following centers and investigators contributed subjects to this trial: United Kingdom: Peter Baldwin MD, Addenbrooke’s Hospital, Cambridge (Regional Coordinating Investigator); Mr Justin Davis, Addenbrooke’s Hospital, Cambridge (Colorectal Surgeon); Emmanuel Huguet, Addenbrooke’s Hospital, Cambridge (HPB/Transplant Surgeon); Ernest Hidalgo MD, Royal Infirmary of Edinburgh, Edinburgh; Owen Cole MD, Nottingham City Hospital, Nottingham; and Kostas Papagiannopoulos MD, St. James University Hospital, Leeds. Germany: Joerg Mezger MD, Vincentius-Kliniken, Karlsruhe; Moritz von Frankenberg MD, Krankenhaus Salem, Heidelberg; Martin Schilling MD, University of Saarland Homburg, Saar; and Christoph Seiler MD, University of Heidelberg, Heidelberg. New Zealand: Jonathan Koea MD, Auckland City Hospital, Auckland (Regional Coordinating Investigator); Michael Rodgers MD, North Shore Hospital, Auckland; and Grant Beban MD, Auckland Gastroenterology Associates, Epsom, Auckland. Australia: Anthony Costello MD, Royal Melbourne Hospital, Parkville, Melbourne, Victoria; Ajay Rane MD, The Townsville Hospital, Townsville, Queensland; Robert Padbury MD, Flinders Medical Centre, Bedford Park, Adelaide, South Australia; and Neil Merrett MD, Bankstown Hospital, Bankstown, Sydney, New South Wales. The contribution of the medical staff, nursing staff, theater staff, and intensive care staff as well as the trial coordinators in each of these centers is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koea, J., Baldwin, P., Shen, J. et al. Safety and Hemostatic Effectiveness of the Fibrin Pad for Severe Soft-Tissue Bleeding During Abdominal, Retroperitoneal, Pelvic, and Thoracic (Non-cardiac) Surgery: A Randomized, Controlled, Superiority Trial. World J Surg 39, 2663–2669 (2015). https://doi.org/10.1007/s00268-015-3106-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3106-5