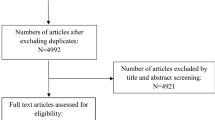
Abstract
Background
Scars are inevitable results of surgical procedures, and prevention of them is still a major problem in the field of cosmetic surgery. Although various studies have been performed on botulinum toxin-A (BoNT‐A) injection for the prevention of hypertrophic scars, the exact mechanism remains unclear.
Methods
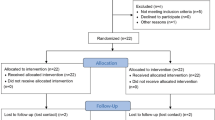
This prospective, double-blinded, randomized study was performed on 19 patients who underwent mammoplasty and abdominoplasty surgery in Razi Hospital from October 2018 to December 2019. Single session of treatment was performed, where XEOMIN was allocated to one half of the scar and 0.9% saline to the control half. 3 and 6 months later, scars were assessed using the modified Stony Brook Scar Evaluation Scale (SBSES).
Results
In total, 19 patients who completed the study were analyzed. mSBSES at the third month (P value < 0.001; 3.34 ± 1.59 vs 1.5 ± 1.36) and the sixth month (P value < 0.001; 4.89 ± 1.83 vs 2.39 ± 1.82) showed a significant difference between the treatment and control groups. In the subset analysis, there was significant difference between BoNT-A and control in all four items including width, height, color, and scar visibility at months 3 and 6, and the BoNT-A-treated sides had higher scores in all items.
Conclusion
BoNT-A has a significant effect on scar prevention due to mammoplasty and abdominoplasty compared to placebo and results in decreased erythema, height, width and reduces incision line visibility. Moreover, its effect increases significantly over time from months 3 to 6.
Level of Evidence II
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.




Similar content being viewed by others
References
Guo X, Song G, Zhang D, Jin X (2019) Efficacy of botulinum toxin type A in improving scar quality and wound healing: a systematic review and meta-analysis of randomized controlled trials. Aesthet Surg J 40:NP273–NP285
Wang D, Qu J, Jiang H, Jiang Y (2019) The safety and efficacy of botulinum toxin for management of scars: a systematic review with meta-analysis and trial sequential analysis. Toxicon 166:24–33
Abedini R, Sasani P, Mahmoudi HR, Nasimi M, Teymourpour A, Shadlou Z (2018) Comparison of intralesional verapamil versus intralesional corticosteroids in treatment of keloids and hypertrophic scars: a randomized controlled trial. Burns 44(6):1482–1488
Wang Y, Wang J, Zhang J, Hu C, Zhu F (2019) Effectiveness and safety of botulinum toxin type A injection for scar prevention: a systematic review and meta-analysis. Aesthet Plast Surg 43(5):1241–1249
Ramos RM, Burland M, Silva JB, Burman LM, Gelain MS, Debom LM, Bec JM, Alirezai M, Uebel CO, Valmier J (2019) Photobiomodulation improved the first stages of wound healing process after abdominoplasty: an experimental, double-blinded. Non-randomized Clinical Trial. Aesthet Plast Surg 43(1):147–154
Dressler D, Saberi FA, Barbosa ER (2005) Botulinum toxin: mechanisms of action. Arq Neuropsiquiatr 63(1):180–185
Scott AB (1980) Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 87(10):1044–1049
Gassner HG, Sherris DA, Otley CC (2000) Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg 105(6):1948–1953
Kim SH, Suh IS, Jeong HS, Lee SJ, Lee JW (2019) Clinical trial to evaluate the efficacy of botulinum toxin type A injection for reducing scars in patients with forehead laceration. Medicine (Baltimore) 98(34):e16952
An MK, Cho EB, Park EJ, Kim KH, Kim LS, Kim KJ (2019) Appropriate timing of early postoperative botulinum toxin type A injection for thyroidectomy scar management: a split-scar study. Plast Reconstr Surg 144(4):659e–668e
Ziade M, Domergue S, Batifol D, Jreige R, Sebbane M, Goudot P, Yachouh J (2013) Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg 66(2):209–214
Shome D, Khare S, Kapoor R (2018) An algorithm using botox injections for facial scar improvement in fitzpatrick type IV–VI skin. Plast Reconstr Surg Glob Open 6(8):e1888
Gassner HG, Brissett AE, Otley CC, Boahene DK, Boggust AJ, Weaver AL, Sherris DA (2006) Botulinum toxin to improve facial wound healing: a prospective, blinded, placebo-controlled study. Mayo Clin Proc 81(8):1023–1028
Kim YS, Lee HJ, Cho SH, Lee JD, Kim HS (2014) Early postoperative treatment of thyroidectomy scars using botulinum toxin: a split-scar, double-blind randomized controlled trial. Wound Repair Regen 22(5):605–612
Kasyanju Carrero LM, Ma WW, Liu HF, Yin XF, Zhou BR (2019) Botulinum toxin type A for the treatment and prevention of hypertrophic scars and keloids: updated review. J Cosmet Dermatol 18(1):10–15
Fearmonti R, Bond J, Erdmann D, Levinson H (2010) A review of scar scales and scar measuring devices. Eplasty 10:e43
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163
Bayat A, McGrouther DA, Ferguson MW (2003) Skin scarring. BMJ 326:88–92
Huang C, Miyazaki K, Akaishi S, Watanabe A, Hyakusoku H, Ogawa R (2013) Biological effects of cellular stretch on human dermal fibroblasts. J Plast Reconstr Aesthet Surg 66:e351–e361
Scala J, Vojvodic A, Vojvodic P, Vlaskovic-Jovicevic T, Peric-Hajzler Z, Matovic D, Dimitrijevic S, Vojvodic J, Sijan G, Stepic N, Wollina U (2019) Botulin toxin use in scars/keloids treatment. Open Access Maced J Med Sci 7(18):2979–2981
Chang CS, Wallace CG, Hsiao YC, Chang CJ, Chen PK (2014) Botulinum toxin to improve results in cleft lip repair: a double-blinded, randomized, vehicle-controlled clinical trial. PLoS ONE 9(12):e115690
Lee SH, Min HJ, Kim YW, Cheon YW (2018) The efficacy and safety of early postoperative botulinum toxin A injection for facial scars. Aesthet Plast Surg 42(2):530–537
Hu L, Zou Y, Chang SJ et al (2018) Effects of botulinum toxin on improving facial surgical scars: a prospective, split-scar, double-blind, randomized controlled trial. Plast Reconstr Surg 141(3):646–650
Acknowledgement
This research has been supported by Tehran University of Medical Sciences and Health Services (97-01-30-38098).
Funding
Funding was provided by Tehran University of Medical Sciences (Grant No. 97-01-30-38098).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was reviewed and approved by the Institutional Review board of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1397.068).
Informed Consent
Patients were informed about the study protocol, and informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abedini, R., Mehdizade Rayeni, N., Haddady Abianeh, S. et al. Botulinum Toxin Type A Injection for Mammoplasty and Abdominoplasty Scar Management: A Split-Scar Double-Blinded Randomized Controlled Study. Aesth Plast Surg 44, 2270–2276 (2020). https://doi.org/10.1007/s00266-020-01916-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-020-01916-7