Abstract
Introduction
The purpose of this study was to evaluate the current perceptions, preferences, and practice of plastic surgeons in Europe regarding breast implant surgery after the controversy on macrotextured implants and BIA-ALCL and the voluntary recall of all biocell implants.
Methods
A survey comprising 15 questions about implant selection and postoperative routines associated with breast augmentation was sent to all society members of the EASAPS.
Results
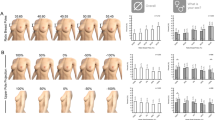
Out of 1473 correspondents, 416 completed the survey with response rate being 28.2%. Countries with less than ten respondents were not included in the analysis. A total of 359 respondents (24.4%) were included in the analysis. Twenty-one respondents (5.8%) had a clinical experience < 5 years, 43 (12%) had 5–10 years’ experience, and 295 (82.2%) had > 10 years’ experience. Regarding the type of implant, only 6.1% would use a macrotextured implant. Fourteen per cent of surgeons would recommend to change a biocell implant in any case, even without symptoms or problems (rupture, seroma, and capsular contracture), 99.7% would send the capsule for histopathological study (99.7%), 98.9% would perform bilateral implant replacement in case of a unilateral problem of rupture, contracture, or seroma, and 80.8% of respondents considered capsulectomy as a technique for managing capsular contracture degree III/IV.
Conclusions
The main conclusion is the heterogenicity of answers and practice, due to the lack of guidelines and scientific evidence on breast implants. Although 22 (6.1%) respondents would use a macrotextured implant (either round or anatomic), 71.6% of respondents considered that there is not enough information for removing macrotextured implants from the market and that they should be allowed to be used unrestrictedly or under close surveillance of the regulatory agencies.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.




Similar content being viewed by others
References
ISAPS International Survey (2019) The international study on aesthetic/cosmetic procedures performed in 2018. https://www.isaps.org/wp-content/uploads/2019/12/ISAPS-Global-Survey-Results-2018-new.pdf. Accessed 11 Feb 2020
Cronin TD, Gerow FJ (1964) Augmentation mammoplasty: a new “natural feel” prosthesis. In: Transactions of the third international congress of plastic surgery (Excerpta medica international congress series No 66). Excerpta Medica, Amsterdam, pp 41–49
Gabriel A, Maxwell GP (2015) The evolution of breast implants. Clin Plast Surg 42:399–404
Market Study Report LLC. (2020) Europe Breast Implant Market analysis research and trends report for 2018–2025. https://www.marketwatch.com/press-release/europe-breast-implant-market-analysis-research-and-trends-report-for-2018-2025. Accessed 11 Feb 2020
Bizjak M, Selmi C, Praprotnik S et al (2015) Silicone implants and lymphoma: the role of inflammation. J Autoimmun 65:64–73
Rupani A, Frame JD, Kamel D (2015) Lymphomas associated with breast implants: a review of the literature. Aesthet Surg J 35:533–544
Fleury EF, Rêgo MM, Ramalho LC et al (2017) Silicone-induced granuloma of breast implant capsule (SIGBIC): similarities and differences with anaplastic large cell lymphoma (ALCL) and their differential diagnosis. Breast Cancer 10:133–140
de Faria Castro Fleury E (2020) Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): an open wound. Aesthet Plast Surg 44:627–629
Groth AK, Graf R (2020) Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and the textured breast implant crisis. Aesthet Plast Surg 44:1–12
De Polo J (2019) Allergan issues worldwide recall of biocell textured breast implants and tissue expanders. https://www.breastcancer.org/research-news/allergen-recalls-biocell-textures-implants. Accessed 11 Feb 2020
FDA (2019) FDA takes action to protect patients from risk of certain textured breast implants; requests Allergan voluntarily recall certain breast implants and tissue expanders from market. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-patients-risk-certain-textured-breast-implants-requests-allergan. Accessed 11 Feb 2020
Alderman A, Pusic A, Murphy DK (2016) Prospective analysis of primary breast augmentation on body image using the BREAST-Q: results from a nationwide study. Plast Reconstr Surg 137:954–960
Keech JA Jr, Creech BJ (1997) Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 100:554–555
FDA (2011) U.S. Food and Drug Administration. FDA update on the safety of sili-cone gel-filled breast implants. June 2011. https://www.fda.gov/media/80685/download. Accessed 28 Feb 2020
Swerdlow SH, Campo E, Pileri SA et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375–2390
Brody GS, Deapen D, Taylor CR et al (2015) Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg 135:695–705
Ramos-Gallardo G, Cuenca-Pardo J, Rodríguez-Olivares E et al (2017) breast implant and anaplastic large cell lymphoma meta-analysis. J Invest Surg 30:56–65
Maxwell GP, Scheflan M, Spear S, Nava MB, Hedén P (2014) Benefits and limitations of macrotextured breast implants and consensus recommendations for optimizing their effectiveness. Aesthet Surg J 34:876–881
Munhoz AM, Clemens MW, Nahabedian MY (2019) Breast implant surfaces and their impact on current practices: where we are now and where are we going? Plast Reconstr Surg Glob Open 7(10):e2466
Benito-Ruiz J (2017) Implantes mamarios en España: resultados de encuesta a cirujanos plásticos. Cir Plást Iberolatinoam 43:239–246
Martin S, McBride M, Khan K (2019) The current UK perspective of breast surgeons on breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Eur J Plast Surg 42:43–48
Coombs DM, Grover R, Prassinos A, Gurunluoglu R (2019) Breast augmentation surgery: clinical considerations. Cleve Clin J Med 86:111–122
Magnusson MR, Connell T, Miroshnik M et al (2019) Breast implant selection: consensus recommendations using a modified Delphi method. Plast Reconstr Surg Glob Open 7(5):e2237
Egeberg A, Sørensen JA (2016) The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 77:255–259
Schenone G, Bernardello E, Lema B (2017) Linfadenopatía axilar por siliconas: revisión. Algoritmo de estudio y tratamiento. Rev Argent Cir Plast 1:0113–0120
Zambacos GJ, Molnar C, Mandrekas AD (2013) Silicone lymphadenopathy after breast augmentation: case reports, review of the literature, and current thoughts. Aesthet Plast Surg 37:278–289
Adams WP Jr, Culbertson EJ, Deva AK et al (2017) Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: experience in 42,000 implants. Plast Reconstr Surg 140:427–431
McGuire PA, Deva AK, Glicksman CA, Adams WP Jr, Haws MJ (2019) Management of asymptomatic patients with textured surface breast implants. Aesthet Surg J Open Forum 1(3):ojz025. https://doi.org/10.1093/asjof/ojz025
FDA (2019) Things to consider before getting breast implants. https://www.fda.gov/medical-devices/breast-implants/things-consider-getting-breast-implants Accessed 1 Mar 2020
Acknowledgements
Statistical analysis and editorial assistant services have been provided by Ciencia y Deporte S.L. We acknowledge all the presidents of the national societies belonging to EASAPS for their help in sending the survey to all members and especially our thanks to the members of the Educational Committee for their support: Dr Jose Carlos Parreira, Dr. Birgit Stark, Dr. Michel Rouif, and Dr. Ivan van Heijningen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical Standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benito-Ruiz, J., Redondo, A. Breast Augmentation Surgery: How Do We Do It? Results of a Joint Survey from European Association of Societies of Aesthetic Plastic Surgery. Aesth Plast Surg 44, 1957–1964 (2020). https://doi.org/10.1007/s00266-020-01846-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-020-01846-4