Abstract
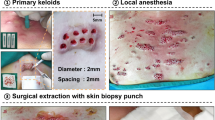
An increasing number of soft tissue filler substances have been introduced to the beauty market outside the U.S. which lackexperimental and clinical data in support of their claim. Ten commercially available filler substances were examined for biocompatibility and durability: 0.1 cc of each substance was injected deep intradermally into the volar forearm of one of the authors and observed for clinical reaction and permanence. At 1, 3, 6, and 9 months the test sites were excised, histologically examined, and graded according to foreign body reactions classification. Collagen (Zyplast) was phagocytosed at 6 months and hyaluronic acid (Restylane) at 9 months. PMMA microspheres (Artecoll) had encapsulated with connective tissue, macrophages, and sporadic giant cells. Silicone oil (PMS 350) was clinically inconspicuous but dissipated into the tissue, causing a chronic foreign body reaction. Polylactic acid microspheres (New-Fill) induced a mild inflammatory response and had disappeared clinically at 4 months. Dextran microspheres (Reviderm intra) induced a pronounced foreign body reaction and had disappeared at 6 months. Polymethylacrylate particles (Dermalive) induced the lowest cellular reaction but had disappeared clinically at 6 months. Polyacrylamide (Aquamid) was well tolerated and remained palpable to a lessening degree over the entire testing period. Histologically, it dissipated more slowly and was kept in place through fine fibrous capsules. Polyvinylhydroxide microspheres suspended in acrylamide (Evolution) were well tolerated, slowly diminishing over 9 months. Calcium hydroxylapatite microspheres (Radiance FN) induced almost no foreign body reaction but were absorbed by the skin at 12 months.Host defense mechanisms react differently to the various filler materials, but all substances— resorbable or nonresorbable—appeared to be clinically and histologically safe, although all exhibit undesirable side effects. Since the mechanism of late inflammation or granuloma formation is still unknown, early histological findings are not useful in predicting possible late reactions to filler substances.
Similar content being viewed by others
References
Alster, T.S., West, T.B.: Human-derived and new synthetic injectable materials for soft-tissue augmentation: Current status and role in cosmetic surgery. Plast Reconstr Surg 105, 2515 (2000)
Amard, P.: PLA (New-Fill) as management of lipoatrophy of the face. Magazin Aesthet Surg 1, 28 (2001)
Athanasiou, K.A., Niederauer, G.G., Agrawal, C.M., Landsman, A.: Applications of biodegradable lactides and glycolides in pediatry. Implant Biomater 12, 475 (1995)
Bendszus, M., Klein, R., Burger, R., et al.: Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. Am J Neurorad 21, 255 (2000)
Bent, A.E., Foote, J., Siegel, S., Faerber, G., Chao, R., Gormley, E.A.: Collagen implant for treating stress urinary incontinence in women with urethral hypermobility. J Urol 166, 1354 (2001)
Bergeret-Galley, C., Latouche, X., Illouz, Y.-G.: The value of new filler material in corrective and cosmetic surgery: DermaLive and DermaDeep. Aesth Plast Surg 25, 249 (2001)
Bigata, X., Ribera, M., Bielsa, I., Ferrandiz, C.: Adverse granulomatous reaction after cosmetic dermal silicone injection. Dermatol Surg 27, 198 (2001)
Burres, S.: Recollagenation of acne scars. Dermatol Surg 22, 364 (1996)
Busso M: Soft tissue augmentation with Radiance FN. Aesthetic Trends & Technologies 2(3): 2003
Cahill, K.V., Burns, J.A.: Volume augmentation of the anophthalmic orbit with cross-linked collagen (Zyplast). Arch Ophthalmol 107, 1684 (1989)
Carr, A., Samaras, K., Burton, S., et al.: A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12, F51 (1998)
Chan, R.W., Titze, I.R.: Viscosities of implantable biomaterials in vocal cord augmentation surgery. Laryngoscope 108, 725 (1998)
Chen AL, Desai P, Adler EM, Di Cesare PE: Granulomatous inflammation after hylan g-f 20 viscosupplementation of the knee: a report of six cases. J Bone Joint Surg (Am) 84-A:1142, 2002
Cheng, N.-X., Wang, Y.-L., Wang, J.-H., Zhang, X.-M., Zhong, H.: Complications of breast augmantation with injected hydrophilic polyacrylamide gel. Aesth Plast Surg 26, 375 (2002)
Cohen SR, Holmes RE (2004) Artecoll: A longlasting wrinkle augmentation material. To be published in Plast Reconstr Surg 113:
DrosbeckHP, Rothstein SS, Gumaer KI, Sherer AD, Slighter RG: J Oral Maxillofac Surg 42:143, 1984
Duffy, D.M.: Injectable liquid silicone: new perspectives. In: Klein, A.W. (ed.) Tissue Augmentation in Clinical Practice: Procedures and Techniques, pp. 237–267. Marcel Dekker, New York (1998)
Duffy, D.M.: The silicone conundrum: A battle of anecdotes. Dermatol Surg 28, 590 (2002)
Duranti, F., Salti, G., Bovani, B., Calandra, M., Rosati, M.: Injectable hyaluronic acid gel for soft tissue augmentation. Dermatol Surg 24, 1317 (1998)
Eppley, B.L., Summerlin, D.-J., Prevel, C.D., Sadove, A.M.: Effects of positively charged biomaterial for dermal and subcutaneous augmentation. Aesth Plast Surg 18, 13 (1994)
ErsekRA, Beisang AA: Bioplastique. A new textured copolymer microparticle promises permanence of softtissue augmentation. Plast Reconstr Surg 87:693, 1991
Feretis, C., Benakis, P., Dimopoulos, C., et al.: Endoscopic implantation of Plexiglas (PMMA) microspheres for the treatment of GERD. Gastrointest Endosc 53, 423 (2001)
Filatov AV, Mirolyubov SN: Contour plasty of maxillofacial soft tissues with biocompatible polyacrylamide gel (Russian). Stomatologiia (Mosk) 77:45, 1998
Flint, P.W., Corio, R.L., Cummings, C.W.: Comparison of soft tissue response in rabbits following laryngeal implantation with hydroxylapatite, silicone rubber and Teflon. Ann Oto Rhino Laryngol 106, 399 (1997)
Friedman, P.M., Mafong, E.A., Kauvar, A.N., Geronemus, R.G.: Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg 28, 491 (2002)
Garcia-Domingo, M.I., Alijotas-Reig, J., Cistero-Bahima, A., Tresserra, F., Enrique, E.: Disseminated and recurrent sarcoid-like granulomatous panniculitis due to bovine collagen injection. J Invest Allergol Clin Immunol 10, 107 (2000)
Geile, D., Zinner, I., Erbel, F., Schaefer, M., Osterholzer, G.: Diagnosis and non-surgical treatment of faecal incontinence in the proctological practice. In: Fruehmorgen, P., Bruch, H.-P. (eds.) Non-Neoplastic Diseases of the Anorectum. Kluwer Academic Publishers, Dordrecht (2001)
Hallen L, Johansson C, Laurent C: Cross-linked hyaluronan (Hylan B gel): an injectable remedy for treatment of vocal fold insufficiency—an animal study. Acta Otolaryngol (Stockholm) 119:107, 1999
Hanke, C.W.: Adverse reactions to bovine collagen. In: Klein, A.W. (ed.) Tissue Augmentation in Clinical Practice, pp. 145–154. Procedures and Techniques. Marcel Dekker, New York (1998)
Kazachkov EL, Fridman AB, Friss SA: Granulomatous pleurisy after mammoplasty induced by polyacrylamide gel. (Russian). Arkh Patol 60:58, 1998
Kinney, B.M., Hughes III, C.E.: Soft tissue fillers: An overview. Aesth Surg J 21, 469 (2001)
Klein, A.W., Elson, M.L.: The history of substances for soft tissue augmentation. Dermatol Surg 26, 1096 (2000)
Kolle FS: Plastic and Cosmetic Surgery. D Appleton, New York, pp 209–230, 1911
Lemperle G, Hoehler H: Granulome nach Unterspritzung von Gesichtsfalten mit Teflon-Paste. In: Hoehler H (Ed.) Plastische und Wiederherstellungschirurgie Schattauer, Stuttgart, pp 335–340, 1975
Lemperle, G., Gauthier-Hazan, N., Lemperle, M.: PMMAmicrospheres (Artecoll) for long-lasting correction ofwrinkles: Refinements and statistical results. Aesth Plast Surg 22, 365 (1998)
Lemperle, G.: Hyaluronsa ure zur Faltenunterspritzung. Arzneimittel-, Therapie-Kritik 3, 635 (2000)
Lemperle, G., Romano, J.J., Busso, M.: Soft tissue augmentation with Artecoll: 10-year history, indications, technique and complications. Dermatol Surg 29, 573 (2003)
Lemperle G, Legaz ME (2004) Biocompatibility of injectable microparticles. Aesth Plast Surg, submitted
Lowe, N.J., Maxwell, C.A., Lowe, P.: DuickMG, Shah K: Hyaluronic acid skin fillers: Adverse reactions and skin testing. J Am Acad Dermatol 45, 930 (2001)
Lupton, J.R., Alster, T.S.: Cutaneous hypersensitivity reaction to injectable hyaluronic acid gel. Dermatol Surg 26, 135 (2000)
Manna, F., Dentini, M., Desideri, P., De Pita, O., Mortilla, E., Mars, B.: Comparative chemical evaluation of two commercially available derivatives of hyaluronic acid (Hylaform from rooster combs and Restylane from streptococcus) used for soft tissue augmentation. J Eur Acad Dermatol Venerol 13, 183 (1999)
Mazzoleni F, Dominici C, Lotti T, et al.: Formacryl: Un nuovo biopolimero al servizio della medicina ‘‘piu un endoprotesi che un filler.’’ Dermatologia 1:13, 2000
MisiekDJ, Kent JN, Carr RF: Soft tissue responses to hydroxylapatite particles of different shapes. J Oral Maxillofac Surg 42:150, 1984
Moscona, R.R., Bergman, R., Friedman-Birnbaum, R.: An unusual late reaction to Zyderm I injections: a challenge for treatment. Plast Reconstr Surg 92, 331 (1993)
Morhenn, V.B., Lemperle, G., Gallo, R.L.: Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol Surg 28, 484 (2002)
Naoum C, Dasou-Plakida D: Dermal filler materials and botulin toxin. Int J Dermatol 40:606, 200
Narins, R.S., Brandt, F., Leyden, J., Lorenc, Z.P., Rubin, M., Smith, S.: A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg 29, 588 (2003)
Neto MS, Passy S: Rellenamiento cutaneo y correccion de las deformidades del rostro con el uso de microesferas de PMMA—un nuevo enfoque. In: Proceedings of the 28th Argentinian Congress of Plastic Surgery, Cordoba, pp 1–8, 1999
O’Connor KW, Lehman GA: Endoscopic placement of collagen at the lower esophageal sphincter to inhibit gastroesophageal reflux: a pilot study of 10 medically intractable patients. Gastrointest Endoscop 34:106, 1988
Olenius, M.: The first clinical study using a new biodegradable implant for the treatment of lips, wrinkles and folds. Aesth Plast Surg 22, 97 (1998)
PannekJ, Brands FH, Schewe J, Senge T: Particle migration following transurethral injection of carbon coated beads for stress urinary incontinence. J Urol 165(Suppl 5):74, 2001
PollackS: Some new injectable dermal filler materials: Hylaform, Restylane, and Artecoll. J Cutan Med Surg 3Suppl 4:S4–S29, 1999
Rapaport, M.J.: VinnikC, Zarem H: Injectable silicone: cause of facial nodules, cellulites, ulceration, and migration. Aesth Plast Surg 20, 267 (1996)
Requena, C., Izquierdo, M.J., Navarro, M., et al.: Adverse reactions to injectable aesthetic microimplants. Am J Dermatopathol 23, 197 (2000)
Rudolph, C.M., Soyer, H.P., Schuller-Petrovic, S., Kerl, H.: Foreign body granulomas due to injectable aesthetic microimplants. Am J Surg Pathol 23, 113 (1999)
Rubin, J.P.: YaremchukMJ: Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: A comprehensive review of the literature. Plast Reconstr Surg 100, 1 (1997)
Saint-Marc, T., Partisani, M., Poizot-Martin, I., et al.: A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS 13, 1659 (1999)
Senet, P., Bachelez, H., Ollivaud, L., Vignon-Pennamen, D., Dubertret, L.: Minocycline for the treatment of cutaneous silicone granulomas. Br J Dermatol 140, 985 (1999)
Shafir, R., Amir, A., Gur, E.: Long-term complications of facial injections with Restylane (injectable hyaluronic acid). Plast Reconstr Surg 106, 1215 (2000)
Spenlehauer, G., Vert, M., Benoit, J.P., Boddaert, A.: In vitro and in vivo degradation of poly(D, L-lactide/glycolide) type microspheres made by solvent evaporation method. Biomaterials 10, 557 (1989)
Tomacic-Jeciz, V.J., Merritt, K., Umbreit, T.H.: Significance of type and the size of biomaterial particles on phagocytosis and tissue distribution. J Biomed Mater Res 55, 523 (2001)
Voy E-D, Mohasseb J: Lipoatrophie als seltene Komplikation nach Auffu llung der Nasolabialfalten mit injizierbaren Implantaten. Magazin Aesth Chir 2:, 2002
Whitehead, W.E., Wald, A., Norton, N.J.: Treatment options for fecal incontinence. Dis Colon Rectum 44, 131 (2001)
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is being reprinted for ISAPS 50th Anniversary Special Issue.
Rights and permissions
About this article
Cite this article
Lemperle, G., Morhenn, V. & Charrier, U. Human Histology and Persistence of Various Injectable Filler Substances for Soft Tissue Augmentation. Aesth Plast Surg 44, 1348–1360 (2020). https://doi.org/10.1007/s00266-020-01827-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-020-01827-7