Abstract
The efficacy of sexual signals in communication is often maximized under specific environmental conditions. Anthropic alterations of these conditions might, thus, negatively affect communication during reproductive behavior. In fossorial animals, inhabiting visually restricted environments, chemical senses are very important. We examined whether climatic episodes of heat-waves with unusual high temperatures may affect the information provided to females by the sexual chemical signals of males of a fossorial reptile, the amphisbaenian Blanus cinereus. The results showed that experimentally heat-altered substrate scent marks of males can still provide information to females about the presence of a male. Females spent more time on males’ scent marks, irrespective of the temperature treatment, than on control clean ones. However, heat-altered scent marks did not seem to convey information about the health state (immune response) of the producer. Females spent more time on unaltered scent marks of healthier males (probably indicating mating preferences for these males), while female preferences for some heat-altered scent marks were not related to size or immune response of the same individual males. Chemical analyses indicated that the overall chemical profile of precloacal secretions (used for scent marking) did not change with increased temperatures. However, the relationship between proportions of some compounds in secretions and males’ immune response found in unaltered secretions was lost in heat-altered ones. We conclude that unusual increased environmental temperatures may decrease the efficacy of underground sexual chemical signals in this amphisbaenian (i.e., a loss of information on male quality), and consequently, may negatively affect sexual selection and reproduction.
Significance statement
It’s crucial to be as successful as possible when you show off to attract a mate. This may depend on how the surroundings affect the effectiveness of your exhibition. So, animals have evolved sexual signals tuned up to their local environmental conditions. A blind fossorial reptile uses substrate scent marks to transmit information to conspecifics. The projected rise in soil temperatures due to climate change could alter this communication. Although brief exposures to high temperatures still allows scent marks to inform females that a male is around, the information related to that male quality is disrupted. This could have detrimental effects on female reproductive success by drastically decreasing their capacity to select a suitable partner. It is unknown if animals could change their sexual signals in order to re-adjust to the rapidly shifting new climatic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The efficacy (i.e., transmission, persistence, etc.) of sexual signals is often maximized under local environmental conditions (Guilford and Dawkins 1991; Endler 1992; Endler and Basolo 1998; Bradbury and Vehrencamp 2011). For example, the coloration of the fan dewlap of some Anolis lizards, used as a sexual display, may differ among species depending on local light conditions to maximize signal detectability in each habitat (Leal and Fleishman 2004). Similarly, in lizards, the characteristics of chemical signals (i.e., proportions of major chemical classes, diversity of compounds, etc.) seem to depend on the environmental conditions (humidity, temperature) in the habitat where each species live (Escobar et al. 2003; Martín et al. 2015b, 2017; Baeckens et al. 2018; Campos et al. 2020). This is important in the current context of global change because some human-induced environmental changes, such as pollution or altered climatic conditions, might deeply affect the efficacy of sexual signals in intraspecific communication, leading to detrimental consequences for fitness. This is because certain essential components of sexual selection, such as sex and species recognition, territorial defense, mate attraction, or partner choice, may change if sexual signals become less effective as a result of pollution (Engström-Öst and Candolin 2006; Lurling and Scheffer 2007; Iglesias-Carrasco et al. 2017) or climate change (Herbert-Read et al. 2010; Møller 2011; Martín and López 2013). Most studies of global change have focused on the direct physiological effects on the individuals or the consequences for their geographical distribution (e.g., Araujo et al. 2006; Diele-Viegas and Duarte Rocha 2018). However, despite the potentially important detrimental effects of global change on sensory ecology and sexual selection of animals (Candolin et al. 2007; Ferrari et al. 2011; Rosenthal and Stuart-Fox 2012; Groot and Zizzari 2019), this is a relatively little explored aspect of the potential negative consequences of global change (Williams et al. 2008; Sih et al. 2011; Stevens 2013).
Chemoreception is widely used in numerous animals for a variety of functions, including intraspecific communication (Müller-Schwarze 2006; Johansson and Jones 2007; Wyatt 2014). For instance, chemoreception plays a major role in social behavior and reproduction in reptiles (for reviews see Mason and Parker 2010; Martín and López 2011). Many reptiles can recognize conspecifics and distinguish between sexes and even between different individuals via chemical cues (e.g., López et al. 2003; Gonzalo et al. 2004; Scott et al. 2015). Additionally, chemical signals could reveal details about the quality of possible rivals and partners (Greene et al. 2001; O’Donnell et al. 2004; Martín and López 2006, 2007; Ibáñez et al. 2012). Chemical substances produced by specific glands in many reptile species appear to be related to various physical and health features of the producer (Martín and López 2006, 2015; Scali et al. 2023). These relationships probably explain how conspecifics can reliably evaluate competitors’ or potential mates’ qualities using these chemical cues, and why individuals can utilize that information to guide their behavior. However, all these important functions of chemical signals in the communication of reptiles and other animals may be altered by human-induced environmental perturbations (Herbert-Read et al. 2010; Martín and López 2013; Iglesias-Carrasco et al. 2018; Groot and Zizzari 2019).
Amphisbaenians, with their morphological and functional adaptations to a fossorial lifestyle, represent a significant and peculiar group of reptiles (Gans 1978; Navas et al. 2004). Their greatly diminished vision, a result of their adaptations to living underground (Gans 1978), makes chemoreception especially helpful for communication (Cooper et al. 1994). Amphisbaenians, for instance, can identify chemical cues from prey (López and Salvador 1992; Semhan et al. 2010; López et al. 2014) or from predators (López and Martín 1994, 2001), and evaluate certain habitat characteristics (López et al. 2002; Martín et al. 2021b). Precloacal or cloacal glands secrete holocrine secretions, which differ in composition between sexes (López and Martín 2005, 2009; Martín et al. 2023). These gland secretions may play a role in intraspecific communication (Cooper et al. 1994) by scent marking substrates inside underground galleries (Jared et al. 1999; Recio et al. 2023). Furthermore, some amphisbaenians possess the ability to use chemical cues to distinguish between familiar and unfamiliar conspecifics (Martín et al. 2020, 2021d), recognize themselves (López et al. 1997; Martín et al. 2020), and discriminate between sexes (Cooper et al. 1994; López and Martín 2009). Scent marks may also be used by females to evaluate the quality of possible mates because chemical variations in secretions reflect a male’s size and health (Martín et al. 2024). Anthropic soil alterations may negatively affect many aspects of the ecology of fossorial animals (Martín et al. 2015a, 2021a; Tibbet et al. 2020). However, it is unknown whether the potential negative effects of global change may have consequences on the efficacy, duration, and information content of underground scent marks, and, therefore, on intraspecific communication and sexual selection of fossorial reptiles.
Here, we simulated an “unusual” heat wave, similar to those expected to become more frequent within a global change scenario (Coumou and Rahmstorf 2012; Stillman 2019; AEMET 2024), and investigated the effect of increased temperatures on the efficacy of sexual chemical signals in the amphisbaenian, Blanus cinereus (López 2015). This is a strictly fossorial reptile species that spends its life buried in sandy substrates covered with leaf litter (Martín et al. 1991) and, as other amphisbaenian species, has probably limited movements and small home ranges (Martín et al. 2021c). Therefore, amphisbaenians are especially susceptible to disturbances in the local soil conditions. Male B. cinereus secrete precloacal secretions (López and Martín 2005, 2009), which are used to produce scent marks that allow chemosensory self- and sex discrimination and mate assessment (Cooper et al. 1994; López et al. 1997; Martín et al. 2024). In this study, we examined: (1) the female responses to males’ substrate scent marks comparing those maintained at ambient (“normal”) temperature with those experimentally heated to simulate a short period of unusual high environmental temperatures. We specifically tested whether, after being deposited and exposed to high temperatures, subterranean scent marks of males may provide the same information on sex identity, morphology, and health state to females compared to unaltered scent marks. Additionally, we analyzed the effects of temperature (2) on the chemical composition of precloacal secretions of males after being deposited on the substrate, and (3) on the signaling potential of these secretions. For this, we examined the relationships between characteristics of chemical profiles and different variables likely related to a male’s “quality” (i.e., body size and immune response), as these traits are apparently preferred by females in a potential mate (Martín et al. 2024). We predicted that high temperatures may disrupt the compounds in male secretions, which will decrease the efficacy of scent marks in communication when signaling the presence and characteristics of a male to females.
Materials and methods
Study animals and maintenance
In an oak woodland of the Guadarrama Mountains (40°43’N, 04°01’W; Navacerrada, Madrid, Spain), we lifted rocks at the beginning of May 2023 and captured adult B. cinereus amphisbaenians (30 males and 20 females) found beneath rocks. On every day of capture, the amphisbaenians were brought in individual cloth bags to the “El Ventorrillo” MNCN-CSIC field station, situated five kilometers away from the capture site. Amphisbaenians were housed in separate indoor terraria (40 × 30 × 30 cm), with a single flat tile (15 × 15 cm) placed over the substrate, which consisted of 5 cm deep layer of loose coconut fiber. A heating cable placed under the terraria with a thermostat set at 22 ºC, which maintained substrate temperature in the cages close to the preferred one for this species (mean ± SE = 20.7 ± 0.5 °C, range = 17.8–23.6 °C; López et al. 1998) and allowed amphisbaenians to achieve an optimal body temperature by thermoregulating inside the substrate. Four times a week, we placed mealworm pupae (Tenebrio molitor) beneath the tiles to provide food to the amphisbaenians. Thus, we allowed amphisbaenians to replicate their behavior observed in the field, where they are constantly buried underground and usually move and stay for long periods under rocks for thermoregulation, foraging, and social interactions (López et al. 1998). To keep soil moisture and provide drinking water, we sprayed water on the substrate daily to prevent it from drying out. We maintained a natural photoperiod since two sizable windows allowed sunlight to enter the room.
The day after capture, we measured with a metallic ruler the snout-vent length of amphisbaenians (SVL; from the tip of the snout to the extreme posterior point of the cloacal flap; males: mean ± SE = 203 ± 3 mm; females: 198 ± 7 mm) and weighed them with a digital balance (body mass; males: mean ± SE = 5.6 ± 0.3 g; females: 4.8 ± 0.7 g). At the end of the experiments, we also estimated a general index of immunocompetence (‘Immune Response’) of males by measuring in vivo the inflammatory response of their skin using the phytohemagglutinin (PHA) injection test (Smits et al. 1999; Martin et al. 2006; Salaberria et al. 2013). We followed previous procedures used in this species (Martín et al. 2024), injecting PHA (0.04 mg/dissolved in 0.02 ml of PBS) at a point of the ventral region of the tail. Then, we calculated the difference in thickness of this point between measurements made before and 24 h after injection using a pressure-sensitive spessimeter (to the nearest 0.01 mm). After the experimental tests concluded and after verifying that the amphisbaenians were in good health, had a normal appearance, and behaved properly, they were released at their capture sites.
Effects of exposing males’ scent marks to high temperatures on site selection by females
To collect the scent of males used in the trials, the first day, half of the males (n = 10 from a total of 20 individuals) were taken from their home cages and temporarily (24 h) individually housed inside closed cylindrical plastic boxes (11 × 10 cm) provided with holes on all sides. Previously, we had placed inside the box one clean round cotton wipe (7 cm diameter) (Cocoon Demak’Up ®, Essity AB, Stockholm, Sweden), which was manipulated with clean forceps and slightly wetted with water. The wipe was covered with coconut fiber substrate (3 cm deep) taken from the home cage of a male, and, then, that male was also placed inside the box. For controls, we placed identical cotton wipes in identical boxes (n = 30), but with clean coconut fiber and without animals. Male and control boxes were placed in the same room where amphisbaenians were housed, under identical environmental conditions for 24 h before being used in tests.
Trials of female preference were performed between the 15th and 21th of May. Early in the morning, prior to each test, we extracted male amphisbaenians from the plastic boxes prepared 24 h before and returned the animals to their home cages for resting and feeding undisturbed for three days. Our goal was to simulate the temperatures experienced by substrate scent marks placed under the rocks used by amphisbaenians in the field under normal conditions, as well as those expected at the same sites under a heat wave. Although, amphisbaenians can temporarily move to colder sites (e.g., deeper depths) when temperature is too high (López et al. 1998), the scent marks remain under the rocks all the time and could be potentially affected by these high temperatures. Therefore, immediately after extracting the males from the boxes, some boxes with wipes (5 that had contained males and 25 controls) were maintained at room ambient temperature (between 17 and 21 °C), while the rest of the boxes with wipes (5 that had contained males and 5 controls) were placed at 30 °C and warmed for 1 h inside a thermal chamber (FRIOCELL FC-B2V-M/FC-404, MMM Group Germany). Boxes were placed in the chamber in random order and position. We chose these temperatures and exposure times based on data from a previous field study (López et al. 1998). During the mating season in the study area, temperatures in the soil under the type of rocks selected by amphisbaenians (i.e., with a rock thickness between 5 and 25 cm) ranged between 15 and 26 °C for most of the day time, with amphisbaenians more often seen under these rocks when temperatures were within their preferred range (see details and graphs in López et al. 1998). Consequently, we considered that during a heat wave 30 °C is a high but “realistic” temperature that could be attained in the soil under these rocks for short periods of time during the hottest hours of the day (between 15 and 16 h GMT). Moreover, climatic models for the study area predict that these high temperatures will be more frequent in the future (AEMET 2024).
We tested females individually in semitransparent plastic testing cages (40 × 30 × 30 cm) with a 5 cm deep clean loose substrate of coconut fiber. We considered three areas in the cages: two areas of equal surface (18 × 30 cm), one at each extreme, where we placed two clean tiles (15 × 15 cm) above the substrate, one in the center of each area. The central area of the cage (4 × 30 cm) was considered as a ‘neutral zone’. We randomly designated one side of the testing cage as a clean control (‘control side’), where we placed under the tile a cotton wipe and coconut fiber taken from one of the control boxes previously prepared that had been maintained at ambient temperature (see above). On the other side of the cage (‘experimental side’), we placed under the tile a cotton wipe and coconut fiber taken, depending on the treatment, from either (1) a control box maintained at ambient temperature (‘Control-Ambient’ treatment); (2) a control box previously heated inside the thermal chamber (‘Control-Warmed’ treatment); (3) a box that had contained a male and had subsequently been kept at ambient temperature (‘Male-Ambient’ treatment) or (4) a box that had contained a male and had subsequently been heated in the thermal chamber (‘Male-Warmed’ treatment) (see above).
All females (n = 20) were tested individually in the four treatments, once per treatment, on different days, with one resting day between trials. The order of presentation and the individual males tested were counterbalanced. Individual males tested for a particular individual female were randomly chosen, but we ensured that the male and the female had been captured in sites located at least 100 m apart to prevent that they might have encountered previously and might remember or prefer a particular scent. Every test began at 9 am GMT by gently taking the focal individual female from her home cage and placing it in the ‘neutral zone’ in the middle of a testing cage. Amphisbaenians quickly buried themselves in the substrate. Then, we noted later the position of the animal in ten occasions (one observation every hour from 10 am to 19 pm GMT). In each survey, we quickly and partially lifted the tiles to monitor the location of the female, which was often in close contact with the cotton wipe. We did not observe females escaping from under the tile as a consequence of this quick observation. If the female was not found under any tile, we could still locate her position in the cage, as at least part of the body was easily visible through the semitransparent bottom and sides of the cage.
We calculated a female ‘Preference Score’ for males (PS) for each treatment as the number of observations in which the female was observed directly under the tile of the ‘experimental side’ (ETn), plus the number of observations where the female was in the ‘experimental side’ of the cage, but not directly under the tile (ESn), multiplied by 0.5 [i.e., PS = Etn + (Esn x 0.5)]. We did not count observations in the ‘control side’ or in the ‘neutral zone’ (where the exact location of the animal could not be determined). Therefore, the possible values of this ‘Preference Score’ ranged from 0 (no observations in the ‘experimental side’ or tile) to 10 (all observations under the tile in the ‘experimental side’).
At the end of each test, females were returned to their home cages, where they were fed and allowed to rest for one day. We discarded the fiber substrates and the cotton wipes used and cleaned the testing cages and tiles with abundant water, leaving them to dry. In the resting day, new boxes with coconut fiber substrate and cotton wipes, empty (n = 30) or with males (n = 10 different individual males from those used in the previous test), were prepared as above. In this way, new clean control or scent marked cotton wipes could be used in further trials conducted 24 h later. At the conclusion of all the trials, the scent marks of every individual male (n = 20) had been randomly used in both treatments (‘Ambient’ and ‘Warmed’) in tests conducted on two different days with wipes from boxes prepared in two different occasions for each male (once every four days).
We used a Generalized Linear Mixed Model (GLMM), with a Poisson distribution and a log-link function, to test whether the ‘Preference Scores’, the response variable, varied between ‘Scent’ type (‘Control’ vs. ‘Male’ treatments) and between ‘Temperature’ treatments (‘Ambient’ vs. ‘Warmed’), both variables as fixed factors. The interaction between ‘Scent’ type and ‘Temperature’ was included in the model as another fixed factor. We also included the body size (SVL) of the focal individual (‘Female Size’) as a covariate and ‘Female Identity’ as a random factor in line with the repeated measures design. We conducted Wald’s chi-square tests for mixed models to estimate the effects of the covariate, the fixed factors, and their interaction. Additionally, we used multimodel inference (Burnham and Anderson 2002; Anderson 2008) to generate a set of candidate models and selected the model with the lowest Akaike value (AIC), using the likelihood ratio test (LRT) as the omnibus test. Models do not differ in plausibility if they do not differ by at least two AIC units (Burnham and Anderson 2002). Therefore, of all the models differing by less than two AIC units from the one with the lowest AIC, we chose the model that included the fewest variables as the most parsimonious. We verified that residuals of the final models met the assumptions of normality and homoscedasticity.
Subsequently, we conducted separate Generalized Lineal Models (GLM) for each ‘Temperature’ treatment, with a Poisson distribution and a log-link function, and multimodel inference as previously described, to test whether female ‘Preference Scores’ for males (response variable) depended on ‘Male Size’ (SVL) or their ‘Immune Response’ (both as continuous covariates), and whether these preferences varied between ‘Temperature’ treatments. Statistical analyses were performed with the Statistica 8.0 software (StatSoft Inc. Tulsa, OK). To minimize observer bias, blinded methods were use when all behavioral data were recorded and/or analyzed.
Effects of exposing male precloacal secretions to high temperatures on the characteristics of chemical profiles and their signaling potential
At the end of the trials, we collected preclocacal secretions from males directly into chromatography high-recovery glass vials (López and Martín 2005, 2009). The secretions were freshly extracted from the same individual males that had been used in the behavioral tests (n = 20) to examine variations in unaltered chemicals in relationship to body size and immune response. Each vial with the secretion of an individual was closed with a septum and a screw cap and directly stored inside a freezer at -20 °C until chemical analyses (‘Ambient’ treatment).
Given the low volume of secretion produced by this amphisbaenian, we had to use additional individual males (n = 10), maintained in identical housing conditions, to examine the effect of exposition to high temperature on compounds in secretions. The secretion from each of these males was collected in an identical open glass vial, which was then placed on a rack at 30 °C for 1 h inside the thermal chamber as above (‘Warmed’ treatment). Afterwards, the vial was extracted from the chamber, closed and stored in a freezer at -20 °C until analyses. Some control empty vials were also subjected to the two treatments to account for potential contaminants resulting from collection methods, heating the vials, or analytical procedures.
Chemical analyses were performed using gas chromatography-mass spectrometry (GC-MS) following procedures previously used for this species (see López and Martín 2005, 2009; Martín et al. 2024). We used Xcalibur software (Finningan Co.) and the NIST/EPA/NIH 2002 mass spectra library to identify and determine relative proportion of compounds (% percentage of the total ion current, TIC, area). Proportions were transformed (Ln[(proportion)/(1-proportion)]) before statistical analyses (Aebischer et al. 1993; García-Roa et al. 2018).
To test for differences in the overall chemical profiles of males depending on the temperature treatment, we conducted permutational multivariate variance analyses (PERMANOVA) (Anderson 2001), based on the Euclidean resemblance matrix (with 999 permutations), using the software PRIMER V6.1.13 (Clarke and Gorley 2006) and PERMANOVA + V1.0.3 (Anderson et al. 2008). To reduce the high number of correlated variables (compounds) to a more manageable set, we conducted a principal component analyses (PCA) using the transformed areas of the 15 compounds found in at least half of individuals to make. The scores derived from the four principal components (PCs) were used as response variables in separated Generalized Linear Models (GLM) with a normal distribution and an identity link function. We used multimodel inference as described above, to test for differences between ‘Temperature’ treatments (fixed factor) and in relationship to ‘SVL’ and the ‘Immune Response’, with these two variables as continuous factors. The initial models also included the two double interactions between the fixed factor and each of the continuous factors.
Results
Effects of exposing male scent marks to high temperatures on site selection by females
The ‘Preference scores’ of individual females varied significantly between ‘Scent’ treatments but did not vary significantly between ‘Temperature’ treatments or with ‘Female Size’, while the interaction between ‘Scent’ type and ‘Temperature’ was not significant (Table 1). Overall, female ‘Preference Scores’ were higher in the two male treatments than in the control ones, independently of the temperature at which the scents were maintained prior to the tests (Fig. 1). Moreover, after multimodel inference, we found that the model including ‘Scent’ type alone had the lowest Akaike value (AIC null model = 311.32; AIC selected model = 308.72; AIC other models > 310.24), and was the only model with a significant omnibus test (LRT, χ21 = 4.60, P = 0.03) and a significant effect of the ‘Scent’ treatment (male, estimate ± SE = 0.1357 ± 0.0636; Wald’s test, χ21 = 4.56, P = 0.033).
The female ‘Preference Scores’ obtained by individual males in the ‘Ambient’ temperature treatment were not significantly correlated with their ‘Preference Scores’ obtained by the same individual males in the ‘Warmed’ temperature treatment with different responding females (Pearson’s correlation: r = -0.11, F1,18 = 0.22, P = 0.64). Thus, we performed separate analyses for each temperature treatment. For males whose scent had been maintained at ambient temperature, the model including ‘Immune Response’ alone had the lowest Akaike value (AIC null model = 74.33; AIC selected model = 70.31; AIC other models > 71.80) and a significant omnibus test (LRT, χ21 = 6.02, P = 0.014). This result indicated a significant positive effect of the ‘Immune Response’ of males on the ‘Preference Scores’ in the ‘Male-Ambient’ treatment (estimate ± SE = 3.0973 ± 1.2774; Wald’s test, χ21 = 5.88, P = 0.015) (Fig. 2a). In contrast, for males whose scent had been heated at 30°C prior to the tests, none of the models had AIC values lower than the null model (AIC null model = 90.86; AIC other models > 91.85) or a significant omnibus test (LRT, χ21 < 1.01, P > 0.31) (Fig. 2b). This indicated that neither ‘SVL’ nor the ‘Immune Response’ could significantly explain the ‘Preference Scores’ in the ‘Male-Warmed’ treatment. Therefore, the scent of males that had a higher immune response obtained higher preference scores from females tested, but only when the scent was kept at ambient temperature, as this effect was not found when the scent was heated before the tests (Fig. 2).
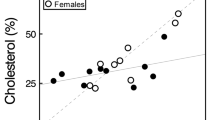
Relationships between the ‘Immune Response’ (mm) of male B. cinereus amphisbaenians and the female ‘Preference Scores’ in tests where females responded to scent-marks of the same males. Scent marks were previously a) maintained at ‘Ambient’ temperature (blue circles) or b) ‘Warmed’ inside a thermal chamber at 30 °C for 1 h (pink triangles)
Effects of exposing male precloacal secretions to high temperatures on the characteristics of chemical profiles and their signaling potential
The most abundant compounds found in the precloacal secretions of male B. cinereus amphisbaenians (Supplementary Table 1) were cholesteryl methyl ether (relative abundance in all samples: mean ± SD = 51.4 ± 8.9%, range = 38.4–75.1%, n = 29) and cholesterol (38.7 ± 7.7%, range = 18.1–49.2%), followed by cholesta-5,7-dien-3-ol, acetate (3.2 ± 1.2%, range = 0.8–5.2%), cholesta-5,7-dien-3-ol (2.3 ± 1.1%, range = 0-5.3%), and squalene (1.6 ± 0.9%, range = 0.4–2.3%).
Males had similar lipophilic compounds independently of the temperature treatment (Supplementary Table 1), and there were no significant differences in the overall chemical profiles (proportion of compounds) (PERMANOVA: pseudo F1,27 = 1.06, P = 0.35) or in the total number of compounds (‘Ambient’: mean ± SD = 15 ± 1 compounds; ‘Warmed’: 14 ± 3; one-way ANOVA on log10 transformed values: F1,27 = 2.21, P = 0.15) between temperature treatments.
The PCA analysis for the transformed areas of the 15 compounds that were found in at least half of individuals, extracted four principal components (PCs) with eigenvalues greater than one, which together accounted for 81.3% of the variance. Each of the PCs described gradients of secretions with different proportions of certain compounds (Supplementary Table 2). For the PC-1, PC-2, and PC-4 no model had an AIC lower than the null model (PC-1: AIC null model = 83.28; AIC other models > 83.96; PC-2: AIC null model = 83.28; AIC other models > 84.35; PC-4: AIC null model = 83.28; AIC other models > 83.89), and in none of the cases the omnibus tests for the “best” models reached statistical significance (LRT, PC-1: χ21 = 1.32, P = 0.25; PC-2: χ21 = 0.93, P = 0.33; PC-4: χ21 = 1.39, P = 0.24). We only found a significant model for the PC-3 scores. This model included the ‘Temperature’ treatment and the interaction between ‘Temperature’ treatment and ‘Immune Response’ of males (AIC null model = 83.24; AIC selected model = 79.12; AIC other models > 79.53), which has a significant omnibus test (LRT, χ22 = 8.12, P = 0.017). This model showed a significant negative relationship of the ‘Immune Response’ of males with the PC-3 scores, which depended on the ‘Temperature’ treatment (Interaction ‘Immune Response’ x ‘Temperature’: estimate ± SE = -5.2172 ± 1.9815; Wald’s test, χ21 = 6.93, P = 0.008), while the effect of the ‘Temperature’ alone did not reach significance (estimate ± SE = 0.6508 ± 0.3842; Wald’s test, χ21 = 2.87, P = 0.09). As the interaction between ‘Temperature’ and ‘Immune Response’ was significant, we calculated separate models for each treatment. In the model for the ‘Ambient’ temperature treatment, the negative relationship between the ‘Immune Response’ and PC-3 scores was significant (estimate ± SE = -5.9936 ± 1.9136; Wald’s test, χ21 = 9.81, P = 0.0017) (Fig. 3). In contrast, in the model for the ‘Warmed’ temperature treatment, this relationship was not statistically significant (estimate ± SE = 2.7593 ± 4.9610; Wald’s test, χ21 = 0.31, P = 0.58) (Fig. 3). Therefore, according to the correlations of the compounds with the PC-3 (Supplementary Table 2), only when secretions were at ambient temperature males with a higher immune response had secretions with higher proportions of cholesta-4,6-dien-3-ol and lower proportions of cholesta5,7-dien-3-ol, acetate, while the proportions of these compounds in secretions did not reflect the immune response of males when secretions were warmed at high temperature.
Relationships between the PC-3 scores representing the relative proportions of certain compounds in precloacal secretions of male B. cinereus amphisbaenians and their ‘Immune Response’ (mm). Secretions were collected from animals maintained at ambient temperature with vials that subsequently were either frozen immediately (‘Ambient’; blue circles) or heated in a thermal chamber at 30°C for 1 h (‘Warmed’; pink triangles) before being frozen until chemical analyses. Arrows indicate the correlations of PC scores with proportion of specific compounds (see Supplementary Table 2)
Discussion
This study found that short episodes of high environmental temperatures, such as those expected from unusual heat-waves, can affect the efficacy of underground chemical signals in the amphisbaenian B. cinereus. However, experimentally heat-altered scent marks still seemed to provide enough information on the presence of a male to females, in comparison with unmarked sites. This could be explained if, irrespective of the temperature treatment, the entire chemical profile (or at least key compounds) of the chemical signals of males remained unaltered. However, exploring the relationships between proportions of some chemicals, male characteristics and female preferences revealed that heat-altered chemical signals seemed to lose information on males’ immune response, potentially affecting females’ ability to make mate choice decisions.
Behavioral tests first showed that female B. cinereus spent more time close to sites scent marked by males than to unmarked sites. This indicated that females could identify these scent marks as produced by a male, or at least by a conspecific, and that the presence of these chemical signals may provide information about the “suitability” of a site for staying there (Recio et al. 2023). Similarly, many types of animals show an attraction for conspecific signals, including chemical cues, when selecting a habitat as a potential indication of the quality of a habitat (reviewed in Mason and Parker 2010; Buxton et al. 2020). Moreover, females might choose sites scent marked by territorial males to increase the opportunities of encountering and mating with such males, while males, in turn, would also benefit from producing scent marks because they would attract those females to their territories (Cole and Smith 1992; Martín and López 2012; Martín et al. 2024). Therefore, it is important that these scent marks remain effective in the environment providing information on the presence of a male for as long as possible (Epple et al. 1980).
We could expect that the characteristics of chemical signals have evolved to maximize their efficacy and durability under the usual environmental conditions prevailing in each habitat (Alberts 1992; Apps et al. 2015; Baeckens et al. 2018). Consequently, environmental climatic alterations can decrease the efficacy of these chemical signals (Herbert-Read et al. 2010; Martín and López 2013; Iglesias-Carrasco et al. 2018). Contrary to our expectations, a short episode of high temperatures, which could be expected from an unusual heat wave, did not seem enough to alter the information conveyed by the scent marks of a B. cinereus male. Females consistently discriminated and preferred scent marks of males irrespective of the temperature to which these marks had been exposed. Similarly, urine scents marks of dingoes (Canis lupus dingo) exposed to the environment for four (but not 33) days could still be classified to age categories by conspecifics (Walker et al. 2024).
Chemical analyses also showed that the overall chemical profile of precloacal secretions of male B. cinereus did not change significantly after a short exposure to high temperatures. However, in other animals, such as the red mason bee (Osmia bicornis), male and female sex-pheromone profiles exhibited changes between low and high temperature regimes (Conrad et al. 2017). In our experiment, we simulated extreme but realistic high temperature conditions in the soil, similar to those expected due to human-mediated climate alterations. However, it is very likely that higher temperatures or longer exposures times will have more pronounced detrimental effects on the compounds of the scent marks. Therefore, the question that arises is whether amphisbaenians could adapt to produce scent marks that can withstand the more frequent, intense and prolonged future episodes of heat waves expected in a global warming scenario (Visser 2008).
In other studies, exposure of scent of lizards to high temperatures has been found to decrease their efficacy in some species but not in others (Martín and López 2013; Iglesias-Carrasco et al. 2018). This phenomenon was explained by the adaptation of species with a more restricted geographical range and stricter ecological requirements to less variable local conditions, while more ubiquitous species that inhabit diverse settings would be adapted to a wide range of environmental conditions, making them more resilient to changes in their surroundings (Iglesias-Carrasco et al. 2018). These findings suggest that some ecological characteristics that result from local adaptation to limited ranges can play a significant role in the evolution of sexual signals. In our study area, we could expect occasional short exposures of substrate scent marks of B. cinereus to the high soil temperature tested in our experiment, well above the species’ preferred temperature (López et al. 1998). However, this amphisbaenian also inhabits warmer areas where high temperatures could be more frequent under normal circumstances. It is possible that the chemical signals of B. cinereus might have evolved to, at least partially, withstand these high temperatures across its entire distribution range.
The main compounds (> 90% of relative proportion) in the precloacal secretions of B. cinereus are two steroids: cholesteryl methyl ether and cholesterol (López and Martín 2005, 2009). These are compounds of high molecular weight (400.68 g/mol and 386.65 g/mol respectively) that are quite stable at high temperatures (i.e., melting points: 86 °C and 148.5 °C; boiling points: 469.7 °C and 360 °C respectively) (Larrañaga et al. 2016). Cholesterol and other steroids are the main compounds in the secretions of many lizards, and are believed to form an unreactive apolar “matrix” that would hold and protect other semiochemicals in the secretion (Escobar et al. 2003; Baeckens et al. 2018), although these steroids may also work as semiochemicals (Martín and López 2007, 2011). In the case of B. cinereus, the abundance of these two stable steroids in its secretions might allow underground scent marks to endure relatively high soil temperatures, maintaining the ability to signal the presence of a male.
In spite of this apparent lack of effect of high temperature on the chemical signals of B. cinereus, more detailed analyses showed that temperatures, however, did significantly alter the information conveyed about male quality. In behavioral tests, females spent more time close to the scent marks of specific individual males, suggesting a preference for the chemical signals of these individual males (Martín et al. 2024). Further examination of male characteristics revealed that females preferred scent marks of males with a higher immune response, probably indicating a better health state, as it was found in previous studies with this and other reptile species (Martín and López 2006; Martín et al. 2024). In a previous experiment, male body size was also important for female B. cinereus, which preferred the scent of larger/older males against smaller/younger males (Martín et al. 2024). However, this preference was not found in the current study, very likely because we used here only males of a similar large size, so that actually there was not enough variability in body size for female choice. Interestingly, the relationship between female preferences and the immune response of a male was only observed when scent marks were unaltered, but not when the scent marks of the same individual males had been previously exposed to high temperatures. Therefore, high temperatures could have altered some compounds that may signal the health state of the scent mark producer, thereby, disrupting the ability of females to choose a high-quality mate. Similarly, in three-spined stickleback (Gasterosteus aculeatus) inhabiting euthrophicated waters, where visibility is reduced due to dense vegetation, mate choice is more costly, and the strength of female selection on male red nuptial coloration is relaxed (Candolin et al. 2007).
Our chemical analyses confirmed that the relationship between the proportion of certain compounds in the chemical signals (precloacal secretions) of male B. cinereus and the immune response of these males was evident in unaltered secretions but was lost after the experimental heat alterations of the secretions. As the volume of secretion produced by these amphisbaenians is low, we could not examine how the compounds changed within the same individual before and after being exposed to high temperatures. However, by comparing secretions of individuals in different treatments, it became evident that disturbances in information content were not due to the simply disappearance of some compounds or their transformation into new ones. Neither the overall chemical profile nor the presence or number of compounds changed after experimental heating. The effect of a short exposure of precloacal secretions to high temperatures is likely to be more complex, perhaps partly due to the potential “protective” effect of the two main steroid compounds. High temperatures might alter each compound differentially in a way that chemical profile of each individual would be no longer confidently correlated with the health state of the producer, as observed in the unaltered secretion. Similarly, in a previous study, exposing the femoral secretions of a lizard to high temperatures decreased the relative abundance of some of the main compounds (e.g., some steroids) but not others (squalene), resulting in overall changes in relative proportions (García-Roa et al. 2018).
We conclude that unusually high temperatures, even with brief exposure times, may have detrimental effects on the information content of male B. cinereus chemical signals, thereby altering females’ ability to choose high quality mates. However, to identify the level of vulnerability of a species’ sensory ecology to the effects of global change, further studies should examine how semiochemical compounds in chemical signals can be disrupted by unfavorable human-induced alterations including changes not only in temperature, drought or humidity, but also in pollution, acidification, etc. Also, we should consider the direct effects of these alterations on the physiology of the receiver, which could affect its chemosensory detection abilities. Finally, new experiments should examine the actual consequences of disruptions in sensory ecology on sexual selection and reproduction, investigating whether animals may respond by modifying the characteristics of their sexual signals or their behavior.
Data availability
Our data are available at: https://doi.org/10.6084/m9.figshare.25562232.
References
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325
AEMET (2024) Generación de escenarios regionalizados de cambio climático. Agencia Estatal de Meteorología, Ministerio para la Transición Ecológica y el Reto Demográfico, Gobierno de España, https://www.aemet.es/es/idi/clima/escenarios_CC
Alberts AC (1992) Constraints on the design of chemical communication systems in terrestrial vertebrates. Am Nat 139:S62–S89
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson DR (2008) Model based inference in the life sciences. Springer, New York
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: guide to software and statistical methods. PRIMER-E Ltd., Plymouth, UK
Apps PJ, Weldon PJ, Kramer M (2015) Chemical signals in terrestrial vertebrates: search for design features. Nat Prod Rep 32:1131–1153
Araújo MB, Thuiller W, Pearson RG (2006) Climate warming and the decline of amphibians and reptiles in Europe. J Biogeogr 33:1712–1728
Baeckens S, Martín J, García-Roa R, Pafilis P, Huyghe K, Van Damme R (2018) Environmental conditions shape the chemical signal design of lizards. Funct Ecol 32:566–580
Bradbury J, Vehrencamp S (2011) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland, MA
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. Springer, New York
Buxton VL, Enos JK, Sperry JH, Ward MP (2020) A review of conspecific attraction for habitat selection across taxa. Ecol Evol 10:12690–12699
Campos SM, Pruett JA, Soini HA, Zúñiga-Vega JJ, Goldberg JK, Vital-García C, Hews DK, Novotny MV, Martins EP (2020) Volatile fatty acid and aldehyde abundances evolve with behavior and habitat temperature in Sceloporus lizards. Behav Ecol 31:978–991
Candolin U, Salesto T, Evers M (2007) Changed environmental conditions weaken sexual selection in sticklebacks. J Evol Biol 20:233–239
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E Ltd., Plymouth, UK
Cole KS, Smith RJF (1992) Attraction of female fathead minnows, Pimephales promelas, to chemical stimuli from breeding males. J Chem Ecol 18:1269–1284
Conrad T, Stöcker C, Ayasse M (2017) The effect of temperature on male mating signals and female choice in the red mason bee, Osmia bicornis (L). Ecol Evol 7:8966–8975
Cooper WE Jr, López P, Salvador A (1994) Pheromone detection by an amphisbaenı́an. Anim Behav 47:1401–1411
Coumou D, Rahmstorf S (2012) A decade of weather extremes. Nat Clim Change 2:491–496
Diele-Viegas LM, Duarte Rocha CF (2018) Unraveling the influences of climate change in Lepidosauria (Reptilia). J Therm Biol 78:401–414
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Am Nat 139:125–153
Endler JA, Basolo AL (1998) Sensory ecology, receiver biases and sexual selection. Trends Ecol Evol 13:416–420
Engström-Öst J, Candolin U (2006) Human-induced water turbidity alters selection on sexual displays in sticklebacks. Behav Ecol 18:393–398
Epple G, Alveario MC, Golob NF, Smith AB (1980) Stability and attractiveness related to age of scent marks of saddle back tamarins (Saguinus fuscicollis). J Chem Ecol 6:735–748
Escobar CM, Escobar CA, Labra A, Niemeyer HM (2003) Chemical composition of precloacal secretions of two Liolaemus fabiani populations: are they different? J Chem Ecol 29:629–368
Ferrari MCO, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP (2011) Intrageneric variation in anti-predator responses of coral reef fishes to ocean acidification: implications of projecting climate change on marine communities. Glob Change Biol 17:2980–2986
Gans C (1978) The characteristics and affinities of the Amphisbaenia. Trans Zool Soc Lond 34:347–416
García-Roa R, Saiz J, Gómara B, López P, Martín J (2018) How to tackle chemical communication? Relative proportions versus semiquantitative determination of compounds in lizard chemical secretions. Ecol Evol 8:2032–2040
Gonzalo A, Cabido C, Martín J, López P (2004) Detection and discrimination of conspecific scents by the anguid slow-worm Anguis Fragilis. J Chem Ecol 30:1565–1573
Greene MJ, Stark SL, Mason RT (2001) Pheromone trailing behavior of the brown tree snake, Boiga irregularis. J Chem Ecol 27:2193–2201
Groot AT, Zizzari ZV (2019) Does climate warming influence sexual chemical signaling? Anim Biol 69:83–93
Guilford T, Dawkins MS (1991) Receiver psychology and the evolution of animal signals. Anim Behav 42:1–14
Herbert-Read JE, Logendran D, Ward AJW (2010) Sensory ecology in a changing world: salinity alters conspecific recognition in an amphidromous fish, Pseudomugil signifer. Behav Ecol Sociobiol 64:1107–1115
Ibáñez A, López P, Martín J (2012) Discrimination of conspecifics’ chemicals may allow Spanish terrapins to find better partners and to avoid competitors. Anim Behav 83:1107–1113
Iglesias-Carrasco M, Head ML, Jennions MD, Martín J, Cabido C (2017) Leaf extracts from an exotic tree affect responses to chemical cues in the palmate newt, Lissotriton helveticus. Anim Behav 127:243–251
Iglesias-Carrasco M, Head ML, Martín J, Cabido C (2018) Increased temperature disrupts chemical communication in some species but not others: the importance of local adaptation and distribution. Ecol Evol 8:1031–1042
Jared C, Antoniazzi MM, Silva JRMC, Freymüller E (1999) Epidermal glands in Squamata: microscopical examination of precloacal glands in Amphisbaena alba (Amphisbaenia, Amphisbaenidae). J Morphol 241:197–206
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289
Larrañaga MD, Lewis RJ, Sr, Lewis RA (2016) Hawley’s condensed chemical dictionary, 16th edn. Wiley, New York
Leal M, Fleishman LJ (2004) Differences in visual signal design and detectability between allopatric populations of Anolis lizards. Am Nat 163:26–39
López P (2015) Culebrilla ciega - Blanus cinereus. In: Salvador A, Marco A (eds) Enciclopedia virtual de Los vertebrados españoles. Museo Nacional de Ciencias Naturales, CSIC, Madrid. http://www.vertebradosibericos.org
López P, Martín J (1994) Responses by the amphisbaenian Blanus cinereus to chemicals from prey or potentially harmful ant species. J Chem Ecol 20:1113–1119
López P, Martı́n J (2001) Chemosensory predator recognition induces specific defensive behaviours in a fossorial amphisbaenian. Anim Behav 62:259–264
López P, Martı́n J (2005) Intersexual differences in chemical composition of precloacal gland secretions of the amphisbaenian Blanus cinereus. J Chem Ecol 31:2913–2921
López P, Martı́n J (2009) Potential chemosignals associated with male identity in the amphisbaenian Blanus cinereus. Chem Senses 34:479–486
López P, Salvador A (1992) The role of chemosensory cues in discrimination of prey odors by the amphisbaenian Blanus cinereus. J Chem Ecol 18:87–93
López P, Salvador A, Cooper WE Jr (1997) Discrimination of self from other males by chemosensory cues in the amphisbaenian (Blanus cinereus). J Comp Psychol 111:105–109
López P, Salvador A, Martín J (1998) Soil temperatures, rock selection and the thermal ecology of the amphisbaenian reptile Blanus cinereus. Can J Zool 76:673–679
López P, Martín J, Cooper WE Jr (2002) Chemosensory responses to plant chemicals by the amphisbaenian Blanus cinereus. Amphibia-Reptilia 23:348–353
López P, Martín J, Cuadrado M (2003) Chemosensory cues allow male lizards Psammodromus algirus to override visual concealment of sexual identity by satellite males. Behav Ecol Sociobiol 54:218–224
López P, Ortega J, Martín J (2014) Chemosensory prey detection by the amphisbaenian Trogonophis wiegmanni. J Herpetol 48:514–517
Lürling M, Scheffer M (2007) Info-disruption: pollution and the transfer of chemical information between organisms. Trends Ecol Evol 22:374–379
Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M (2006) Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol 20:290–299
Martín J, López P (2006) Links between male quality, male chemical signals, and female mate choice in Iberian rock lizards. Funct Ecol 20:1087–1096
Martín J, López P (2007) Scent may signal fighting ability in male Iberian rock lizards. Biol Lett 3:125–127
Martín J, López P (2011) Pheromones and reproduction in reptiles. In: Norris DO, Lopez KH (eds) Hormones and reproduction of vertebrates, reptiles, vol 3. Academic, San Diego, CA, pp 141–167
Martín J, López P (2012) Supplementation of male pheromone on rock substrates attracts female rock lizards to the territories of males: a field experiment. PLoS ONE 7:e30108
Martín J, López P (2013) Effects of global warming on sensory ecology of rock lizards: increased temperatures alter the efficacy of sexual chemical signals. Funct Ecol 27:1332–1340
Martín J, López P (2015) Condition-dependent chemosignals in reproductive behavior of lizards. Horm Behav 68:14–24
Martín J, López P, Salvador A (1991) Microhabitat selection of the amphisbaenian Blanus cinereus. Copeia 1991:1142–1146
Martín J, López P, Gutiérrez E, García LV (2015a) Natural and anthropogenic alterations of the soil affect body condition of the fossorial amphisbaenian Trogonophis Wiegamnni in North Africa. J Arid Environ 122:30–36
Martín J, Ortega J, López P (2015b) Interpopulational variations in sexual chemical signals of Iberian wall lizards may allow maximizing signal efficiency under different climatic conditions. PLoS ONE 10:e0131492
Martín J, Zamora-Camacho FJ, Reguera S, López P, Moreno-Rueda G (2017) Variations in chemical sexual signals of Psammodromus algirus lizards along an elevation gradient may reflect altitudinal variation in microclimatic conditions. Sci Nat 104:16
Martín J, Raya-García E, Ortega J, López P (2020) How to maintain underground social relationships? Chemosensory sex, partner and self recognition in a fossorial amphisbaenian. PLoS ONE 15:e0237188
Martín J, Barja I, Rodríguez-Ruiz G, Recio P, García LV (2021a) Soil pollution by heavy metals correlates with the levels of faecal glucocorticoid metabolites of a fossorial amphisbaenian reptile. Conserv Physiol 9:coab085
Martín J, Ibáñez A, Garrido M, Raya-García E, López P (2021b) Chemical cues may allow a fossorial amphisbaenian reptile to avoid extremely saline soils when selecting microhabitats. J Arid Environ 188:104452
Martín J, Ortega J, García-Roa R, Jiménez-Robles O, Rodríguez-Ruiz G, Recio P, Cuervo JJ (2021c) Going underground: short- and long-term movements may reveal the fossorial spatial ecology of an amphisbaenian. Mov Ecol 9:14
Martín J, Raya-García E, Ortega J, López P (2021d) Offspring and adult chemosensory recognition by an amphisbaenian reptile may allow maintaining familiar links in the fossorial environment. PeerJ 9:e10780
Martín J, Rodríguez-Ruiz G, Cuervo JJ, López P (2023) Intersexual and body size-related variation in chemical constituents from feces and cloacal products involved in intraspecific communication of a fossorial amphisbaenian. PeerJ 11:e15002
Martín J, Rodríguez-Ruiz G, Navarro-Castilla A, Barja I, López P (2024) Blind date: female fossorial amphisbaenians prefer scent marks of large and healthy males. Integr Zool (Published Online. https://doi.org/10.1111/1749-4877.12802)
Mason RT, Parker MR (2010) Social behavior and pheromonal communication in reptiles. J Comp Physiol A 196:729–749
Møller AP (2011) When climate change affects where birds sing. Behav Ecol 22:212–217
Müller-Schwarze D (2006) Chemical ecology of vertebrates. Cambridge University Press, Cambridge
Navas CA, Antoniazzi MM, Carvalho JE, Chaui-Berlink JG, James RS, Jared C, Kohlsdorf T, Pai-Silva MD, Wilson RS (2004) Morphological and physiological specialization for digging in amphisbaenians, an ancient lineage of fossorial vertebrates. J Exp Biol 207:2433–2441
O’Donnell RP, Ford NB, Shine R, Mason RT (2004) Male red-sided garter snakes, Thamnophis sirtalis parietalis, determine female mating status from pheromone trails. Anim Behav 68:677–683
Recio P, Rodríguez-Ruiz G, Sannolo S, Cuervo JJ, López P, Martín J (2023) Conspecific scent-marks may influence underground site selection by a fossorial reptile. Behav Ecol Sociobiol 77:29
Rosenthal GG, Stuart-Fox D (2012) Environmental disturbance and animal communication. In: Candolin U, Wong BBM (eds) Behavioural responses to a changing world: mechanisms and consequences. Oxford University Press, Oxford, pp 16–31
Salaberria C, Muriel J, de Luna M, Gil D, Puerta M (2013) The PHA test as an indicator of phagocytic activity in a passerine bird. PLoS ONE 8:e84108
Scali S, Sacchi R, Gozzo E, Chiesa S, Coladonato AJ, Zuffi MAL, Mangiacotti M (2023) The size of a smell: assessment of rival’s relative size from femoral secretions in the common wall lizards, Podarcis muralis (Laurenti, 1768). Behav Ecol 34:306–313
Scott ML, Llewelyn J, Higgie M, Hoskin CJ, Pike K, Phillips BL (2015) Chemoreception and mating behaviour of a tropical Australian skink. Acta Ethol 18:283–293
Semhan RV, Halloy M, Montero R (2010) Chemical prey discrimination of termites in Amphisbaena heterozonata (Reptilia: Squamata): a learned trait? J Herpetol 44:489–492
Sih A, Ferrari MCO, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387
Smits JE, Bortolotti GR, Tella JL (1999) Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct Ecol 13:567–572
Stevens M (2013) Sensory ecology, behaviour, and evolution. Oxford University Press, Oxford
Stillman JH (2019) Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 34:86–100
Tibbett M, Fraser TD, Duddigan S (2020) Identifying potential threats to soil biodiversity. PeerJ 8:e9271
Visser ME (2008) Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc R Soc Lond B 275:649–659
Walker BJJ, Letnic M, Bucknall MP, Watson L, Jordan NR (2024) Male dingo urinary scents code for age class and wild dingoes respond to this information. Chem Sens 49:bjae004
Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G (2008) Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol 6:e325
Wyatt TD (2014) Pheromones and animal behaviour. Chemical signals and signatures. Cambridge University Press, Cambridge, UK
Acknowledgements
We thank two anonymous reviewers for their helpful comments and “El Ventorrillo” MNCN-CSIC Field Station for providing us with access to their facilities.
Funding
Open access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was funding by the Spanish Ministerio de Ciencia e Innovación grant PID2021-122358NB-I00 (MCIN/AEI/https://doi.org/10.13039/501100011033 and ERDF A way of making Europe).
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
JM and PL designed the experiment and the methodology; JM, PL, AN-C, AdlC and JJC collected the data; JM analyzed the data and wrote the first draft. All authors contributed critically to the draft and gave their final approval for publication.
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the use of animals were followed. The captures of amphisbaenians were performed under license granted by the “Dirección General de Biodiversidad y Recursos Naturales”, Comunidad Autónoma de Madrid (Spain) (Ref. 10/170740.9/21). The experimental procedures were supervised by the “Comisión Ética de Experimentación Animal (CEEA)’’ of the Museo Nacional de Ciencias Naturales, CSIC, and the “Comité de Ética” of the Spanish National Research Council (CSIC), and were approved by the “Area de Protección Animal” of the Comunidad de Madrid Government (Code: PROEX 140.0/23).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by T. Madsen.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martín, J., Navarro-Castilla, Á., de la Concha, A. et al. Heat-altered scent marks of males of a fossorial reptile still allow recognition by females but lose information on male quality. Behav Ecol Sociobiol 78, 77 (2024). https://doi.org/10.1007/s00265-024-03496-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03496-x