Abstract
Camouflage represents an important component of self-protection when animals cannot easily evade predators and is often altered by behavioural responses to a predation threat. The cryptic plumage of many precocial chicks inspired early theoretical work on camouflage mechanisms, but so far, limited efforts have gone towards empirically testing the crypsis of chick plumage properties in their natural environment. We studied background matching and hiding behaviour in precocial snowy plovers Charadrius nivosus in Bahia de Ceuta, Northwest Mexico. This ground-nesting wader breeds in sparsely vegetated open habitats such as salt flats and sandy beaches. The open habitats provide a challenge for young chicks to evade predator detection. Examining background matching of wild chicks for luminance, pattern and colouration at their hiding spots, we found that chicks matched the luminance of their chosen spot better than at unchosen nearby spots. Pattern and colouration matching were age-related, with the plumage of older chicks matching their hiding spots better than those of recently hatched chicks. This suggests that with increasing mobility, chicks may be better able to find hiding places that optimise camouflage. Finally, we found that chicks were more likely to hide in soil cracks than expected by chance, suggesting that chicks chose these soil features in a barren landscape as preferred hideouts. We conclude that the cryptic plumage is an understudied but essential part of the anti-predator repertoire of precocial chicks. The plumage most likely works hand-in-hand with the anti-predator behaviours of chicks and their parents to increase survival chances of precocial young.
Significance statement
Many chicks rely on effective camouflage to evade predators and survive until fledging. We studied how plumage characteristics and behavioural choices enable snowy plover chicks to hide effectively from approaching predators in an open landscape. These chicks leave their nest scrapes shortly after hatching, relying on their cryptic plumage for several weeks to evade predator detection. We found that chicks chose hiding spots where their plumage had a higher match in luminance and, for older chicks, a higher match in pattern and colouration than at adjacent spots. When available, chicks chose to hide in small cracks that appeared in the soil from the evaporation of moisture. This study represents the first quantitative characterisation of cryptic chick plumage features in a natural population. Our results demonstrate that plumage and behavioural responses jointly contribute to the effective camouflage of small chicks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Camouflage is a widespread strategy employed by predators and prey alike to evade detection (e.g. Cuthill 2019). Effective visual camouflage leads to crypsis that increases survival and offers striking examples of natural selection (Stevens and Ruxton 2019). Camouflage has been studied across a large variety of taxa and environments, resulting in the characterisation of several mechanisms, such as disruptive colouration, countershading, and background matching, to enhance crypsis (Stevens and Merilaita 2009). For disruptive colouration, highly contrasting patterns create false edges and disguise the outline of animals. For countershading, animals feature dark colours on body parts facing illumination and light colours on body parts that are usually shaded. For background matching, an animal’s appearance imitates the surrounding background by reducing luminance, colour, or pattern differences.
In addition to morphology, behaviour can improve the effectiveness of a camouflage strategy (Stevens and Ruxton 2019). Background choice is a simple behavioural adaptation to reduce detectability. For example, for some species of Sphingonotus grasshoppers, Podarcis lizards, and Carcinus shore crabs, individuals choose substrates that match their own appearance and improve camouflage (Marshall et al. 2016; Camacho et al. 2020; Twort and Stevens 2023). Heterogeneous backgrounds can optimise camouflage, with some species preferring to hide on visually complex backgrounds (Xiao and Cuthill 2016). Hiding in or near specific structures and vegetation can reduce detectability, in addition to fine-scale positioning behaviour (Hancock et al. 2023). Here, detectability is reduced through optimal alignment of the body to the textures of their microhabitat that minimise ‘true’ edges by creating ‘false’ edges (Kang et al. 2012; Baños-Villalba et al. 2017). Finally, some species may modify their microhabitats for improved crypsis, such as ground-nesting birds choosing nesting materials that conceal the eggs (Stevens et al. 2017; Gómez et al. 2018): Kittlitz’s plovers Charadrius pecuarius bury their eggs under pebbles (Troscianko et al. 2016a) and blue-footed boobies Sula nebouxii soil their eggs to reduce conspicuousness (Fernando Mayani-Parás et al. 2015).
Camouflage often exploits cognitive limitations in the processing of visual signals by the viewer, in this case, the predator. Visual camouflage acts by minimising the signal-to-noise ratio at various stages of visual perception and recognition, deceiving by manipulating the visual information transmitted to the viewer, which alters their judgement regarding the presence/absence, edibility, or profitability (Merilaita et al. 2017). Therefore, behavioural adaptations associated with camouflage are intrinsically linked with cognitive decision-making processes. Animals must be able to assess how well hidden they are on a given substrate in order to change the microhabitat or their appearance (Skelhorn and Rowe 2016). This may involve a basic cognitive appreciation of their own appearance although the cognitive processes underlying background matching are not well understood. Several studies suggest that animals can adjust their appearance to the surrounding environment. Some animals, such as cephalopods or Bradypodion chameleons, can change their colouration or pattern to avoid detection by visual predators (Hanlon et al. 2008; Stuart-Fox et al. 2008). Others, such as red chameleon prawns Hippolyte varians, select colour-matching substrates when presented with a choice but also change their colour over time in response to a new substrate (Green et al. 2019).
Many animals undergo changes in their own appearance during ontogeny or the annual cycle that will affect their camouflage efficacy (Wilson et al. 2007; Nokelainen et al. 2019). Overall, variation in appearance often decreases during development with an emerging generalist phenotype that provides a basic level of camouflage in multiple habitats (Nokelainen et al. 2019). These ontogenetic changes may also be accompanied by changes in individual preferences for background selection (Green et al. 2019). Finding an optimal background for hiding when a predator suddenly approaches depends on mobility and locomotive abilities. The more mobile an organism, the more effective the escape response and the larger the range of available micro-habitats. The developmental stage often does have a strong impact on mobility. In vertebrates, juveniles are less mobile than mature adults meaning that juveniles have more difficulties in evading predators than adults. This makes effective camouflage more important during this stage. However, development also impacts cognition. In many animals, cognitive capabilities take considerable time and experiences to develop during ontogeny and are not fully expressed in juveniles yet (Gómez 2005).
Here we investigate choice of the hiding spot and background matching in young precocial snowy plover Charadrius nivosus chicks. As with most wader species, snowy plovers are ground-nesting birds that nest in open habitats where effective camouflage of the eggs and the chicks often provides the last line of defence to evade predation (Colwell et al. 2011; Stoddard et al. 2016; Rohr et al. 2021). Camouflage of wader eggs and nests has been studied intensively (e.g. Nguyen et al. 2007; Colwell et al. 2011; Skrade and Dinsmore 2013; Troscianko et al. 2016a, b; Gómez et al. 2016, 2018; Stoddard et al. 2016; Stevens et al. 2017), revealing that the colouration of the eggshell provides effective protection from predation (Skrade and Dinsmore 2013; Troscianko et al. 2016a, b; Stoddard et al. 2016). Nesting waders adaptively choose and sometimes even alter the microhabitat of nests to improve camouflage (Troscianko et al. 2016b; Stevens et al. 2017; Gómez et al. 2018). By contrast, the camouflage of the plumage of wader chicks is much less understood and has thus far not been studied in detail (Ferns 2003; Rohr et al. 2021). When approached by predators or humans, young wader chicks typically crouch to the ground and stay motionless in their hiding spots (Colwell et al. 2007). A recent study suggested that the neoptile feathers of chicks help to make the outline of chicks harder to detect (Rohr et al. 2021), thereby enhancing camouflage. However, the disguise of the outline is likely not the main mechanism for camouflage of the chick. The mottled plumage of wader chicks has featured prominently in early work on adaptive colouration. Hugh Cott (1940) referred to the cryptic plumage of wader chicks and their crouching behaviour when discussing effective concealment, where he used woodcock Scolopax rusticola and common ringed plover Charadrius hiaticula chicks to describe the principles of disruptive colouration and countershading.
We tested whether young (1–6 day old) snowy plover chicks choose a hiding spot that improves their camouflage when approached by a simulated predator. First, we tested how well the chick matched the background of their chosen hiding spot. Specifically, we tested whether the chick plumage had a higher match in luminance, pattern, and/or colouration to the background at their hiding spot than at two other possible hiding places adjacent to the chosen spot. Because plover chicks become more mobile with age, we examined whether camouflage (and particularly background matching) of the chicks would improve with age, as young chicks will simply crouch to the ground immediately, whereas older and more mobile chicks may be able to quickly move to a better hiding location where their body matches the background better. To confirm potential age effects linked to mobility or cognition, we digitally cropped real chick images and transferred them to new backgrounds to compare camouflage properties of the chicks at their hiding spots with those of chosen backgrounds of conspecifics of the same or different age class. Second, we investigated whether chicks prefer certain habitat features for hiding. The brood habitat of snowy plovers is very open with sparse vegetation, leaving few places to hide other than for the large cracks in the desiccated soil (Fig. 1), which increases the heterogeneity of the background and plausibly could provide hiding places for small chicks. We therefore tested whether chicks were more likely to associate with such cracks than expected by chance.
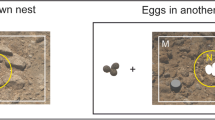
Example of camouflage pictures taken of snowy plover chicks, the calculation of the region-of-interests (ROIs), and crack coverage. a Picture with the chick found in the original chosen hiding spot (scene 1). The white polygon shows the selected ‘chick ROI’, with legs, rings, and feather contours excluded. b The same background picture without the chick (scene 2). The outline of the cropped chick represents the inner border, and the oval around the cropped chick represents the outer border of the ‘in-situ’ microhabitat ROI (centre). The shape of the microhabitat ROI was then shifted to the left and right side (‘shifted’ background positions) to assess whether the chick optimises background matching at its hiding spot. c Background without chick and ROI’s. d Example of the binary image used for the quantification of crack pixels (white) for images with the polymer standard excluded (blue)
Materials and methods
Study site and fieldwork
We conducted fieldwork on breeding snowy plovers at Bahía de Ceuta, Sinaloa, Mexico, from April to July 2017–2019. The breeding site consists of sparsely vegetated salt flats surrounded by mangroves (Cruz-López et al. 2017). The local predator community is diverse and includes coyotes (Canis latrans), feral dogs (Canis familiaris), racoons (Procyon lotor), bobcats (Lynx rufus), opossums (Didelphis virginiana), crested caracaras (Caracara cheriway) (Stoddard et al. 2016) whereas predation by other avian predators of snowy plover chicks at other sites such as gulls and terns is rare at our site. To sample chicks for our study, we first searched intensively for nests using binoculars and spotting scopes from within mobile hides to minimise disturbance (Székely et al. 2008; Eberhart-Phillips et al. 2020). Once a clutch was found, we estimated the hatching date by floating the eggs in a jar of lukewarm water, assuming an incubation period of 25 days (Carmona-Isunza et al. 2017; Plaschke et al. 2019). We checked nests regularly at intervals of three to five days until they reached an age of 20 days, after which we visited at least once every 48 h until hatching or failure. Snowy plovers typically produce three-egg clutches that hatch after incubation over a period of 12 to 48 h (Page et al. 2009; Eberhart-Phillips et al. 2020). To facilitate unique identification throughout ontogeny, we ringed chicks with an alphanumeric metal band – timing the first capture before the precocial chicks left the nest scrape within a few hours of hatching. To examine the camouflage of chicks and how it relates to their hiding spots, we took pictures of chicks that had left the nest scrape. To ensure basic mobility of chicks, we only targeted families that had moved at least 50 m away from the nest scrape. During the three seasons, we photographed 71 individuals and used 44 individuals for analysis (i.e., 27 individuals were excluded because of incorrect exposure, focus, multiple chicks in one picture scene, or vegetation obstructing the appropriate view of the chick). Most data from 2018 were excluded because of missing photographs of the background without the chick. To take chick pictures, two people approached plover families in mobile hides and stopped at a distance of ~ 100 to 200 m from the brood. One person then exited the hide and acted as a potential predator approaching the brood, being careful not to step on the chicks. At the same time, the other observer attempted to keep track of the chicks and determine where they were hiding to direct the ‘predator’ towards their location. When searching for the chicks, we took care to walk carefully and leave as few footprints as possible to avoid changes to the soil that may have altered our conclusion about the preferred hiding spot of the chicks. To take photos, we used a Nikon D7000 camera converted to full spectrum including the UV range (Optic Makario GmbH, Germany). Based on available calibration data (Troscianko and Stevens 2015), we used a Nikkor macro 105 mm lens because this lens also transmits light at low wavelengths. We took all photos during the daytime at least one hour after sunrise and one hour before sunset (Stoddard et al. 2016). We took all photographs directly from above with a tripod at a height of 130 cm and placed a 25% reflectance standard (Zenith Polymer 382 Diffuse Reflectance Standard, Sphereoptics, Germany) in the view field of the camera to allow standardisation of light conditions for all pictures of microhabitat and chicks in their hiding places. As our study involved focal animals in the field, it was not feasible to record data blind.
For each chick, we took photos in two scenes. For scene 1 pictures, we photographed the chick exactly as it was found. We then gently removed the chick, placed it in a bird bag and photographed the hiding place without the chick, which will be referred to as scene 2 pictures. We set the aperture to f/8, ISO 400 for all pictures, and stored the images in RAW format. We used the exposure bracketing setting (e.g., 1 EV interval, from -1 EV to + 1 EV), which produces three pictures with different exposures to ensure that we had at least one picture with appropriate exposure. For each scene, we took separate pictures in visible and UV light spectra using custom-made filters provided by Optic Makario GmbH, Germany: a UV pass filter for the UV spectrum and a UV/IR blocking filter ‘IR – Neutralisationsfilter NG’ for the visible spectrum. Changing between visible and UV modes required a manual change of the filters, which often resulted in tiny movements of the chicks and prevented the appropriate alignment of the UV and visual spectra pictures. Therefore, we excluded all UV Pictures and only analysed images in the visual spectrum.
Image analysis
We used Multispectral Image Calibration and Analysis (‘mica’) Toolbox Plugin version 1.11 (Troscianko and Stevens 2015) for ImageJ version 1.51 k (Schneider et al. 2012) to analyse each picture with the optimal exposure. We produced multispectral images standardised and linearised against the 25% reflectance standard, and converted them to predator vision (Troscianko and Stevens 2015). Because of the experimental approach, the local predator community, and the lack of appropriate UV images, we used a human trichromatic vision model (Stockman and Sharpe 2000) to represent the sensory system of predators.
Region selection
To select the region-of-interest (henceforth ‘ROI’) of the chick in the scene 1 picture, we manually drew closed polygons closely matching the contours of the body, excluding the legs, from now on called ‘chick ROI’ (Fig. 1a). We also excluded fringed contour feathers of the chick outline to avoid false data due to background shining through feathers (Rohr et al. 2021). For the scene 2 image, we then selected the microhabitat ROI for background matching by inserting the chick contour outline (including legs, rings and fringed feathers), placed an oval of customised size (1300 × 1800 pixels) around the chick contour, and removed the area within the chick contour outline from inside the oval. This oval (‘in-situ’ or ‘hiding’ background position) provided the microhabitat ROI of the hiding spot without shadows of the chicks, as these shadows could not be replicated adequately during subsequent digital image manipulations. We then shifted the shape of the microhabitat ROI on the image left and right of the ‘in-situ’ microhabitat, keeping the chick shape aligned with the original posture in the ‘in-situ’ hiding spot (‘shifted’ background positions, Fig. 1b).
Background matching
The age of chicks participating in our background matching experiment varied from 0 to 3 days. We categorised the chicks into two age groups: ‘hatch day’ (0 days old) and ‘after hatch day’ (more than 0 days old). We used pattern, luminance and colour differences to quantify the matching of the chick to their ‘background habitat’ and compared those parameters of the ‘chick ROI’ against the three different microhabitat ROIs (one measurement for the ‘in-situ’ and two measurements for the ‘shifted’ position in their background habitats). We followed previously published methodology (Troscianko and Stevens 2015; Troscianko et al. 2017) for picture analysis. Luminance ranged from 0 to 100%, classified into 20 different bins and luminance distribution differences were calculated as the absolute difference in pixel count at each level between chick and background. To quantify pattern differences, we used a granularity pattern analysis based on Fast-Fourier bandpass filtering at 17 spatial scales and for each scale pattern, energy was calculated with the standard deviation of the luminance values. We compared the absolute difference in pattern energy in each spatial scale of chick and background. This method considers size, spacing, and contrast at any given spatial scale. Colour differences are based on ‘just-noticeable-differences’ (JND), which indicates whether the predator visual system, in our case humans, could discriminate two colours from each other. The comparison between chicks and backgrounds worked as follows: First, we calculated the pattern, luminance, and JND difference between chick (i.e., ‘chick ROI’) and different background microhabitat (i.e., ‘in-situ’ and ‘shifted’ background positions) values. We then compared the ‘in-situ’ differences with the ‘shifted’ differences, with smaller differences indicating better camouflage, i.e. a higher match between chick and background.
Digital transfer experiment
As chicks age, they become more mobile, which may influence their hiding behaviour. Particularly, within the first 24 h after hatching, chicks are often not yet fully mobile and will crouch faster and in a more restricted area than older chicks when a predator approaches (Page et al. 2009). In addition, the plumage of freshly hatched chicks is often not fully dry, which may alter the camouflage properties of the neoptile feathers. To test the robustness of the age effects, we conducted a follow-up digital swap experiment. In this analysis, we utilised the same image analysis method as in the background choice experiment. However, instead of comparing the ‘chick ROI’ from scene 1 with its own ‘in-situ’ and ‘shifted’ background habitat positions from scene 2, we compared the ‘chick ROI’ with ‘in-situ’ backgrounds of conspecific chicks from scene 2 belonging to either the same or a different age category, which we labelled ‘background age’.
Association of chicks with cracks
Shallow cracks are a common feature of the salt flat substrate that swiftly develops on the surface when the soil dries out (Fig. 1c). We tested whether chicks were more likely to use these cracks as hiding places than expected by chance. We found cracks on images of 25 chicks, with a chick age range from 0–5 days. For the subsequent analysis, we adapted the chick ROI to also include legs but not the area of fringed feathers with the software ImageJ. The area covered by the reflectance standard was excluded from the image analysis (blue region in Fig. 1d). Crack pixels were darker than the other soil and hence could be identified based on hue, saturation and brightness properties within each image. However, because of high variation between images, we adjusted the selection individually in each image using the function ‘Color Threshold’ in Image J (see Fig. 1d). By adjusting thresholds for hue, saturation, and brightness we were able to robustly classify crack (value = 0) and soil (value = 1) pixels and create a binary image (Fig. 1d). To test whether chicks were more likely to hide in cracks than in other areas we first calculated the percentage of the ‘chick ROI’ overlapping with crack pixels. For this, we fully aligned scene 1 and 2 pictures using a custom script made with Python (version 3.8.5, van Rossum and Drake 2009), in conjunction with ORB keypoint matching (Rublee et al. 2011) and RANSAC outlier (Fischler and Bolles 1981) to locate the original chick position on the scene 2 images (Fig. 1d). We then calculated random overlaps between ‘chick ROI’ and crack pixels of the scene 2 background through 1000 simulations. For the simulation, we allowed random rotation (0—359°) and random placement of the chick shape as long as the simulated chick did not protrude the image boundaries or overlap with the grey standard. The frequency distribution of the simulated overlaps provides an individual description of the habitat of each image regarding the size and coverage of the occurring cracks. We predicted that the overlap should be larger than expected by chance if chicks chose cracks for hiding.
Statistical analysis
Background matching
We first tested whether the chosen hiding spot provided a better match for the chick to the background for pattern, luminance, and colour. We applied natural logarithmic transformation to pattern but did not transform luminance and JND values as they were normally distributed. We examined the three response variables in separate linear mixed models (LMM) via the ‘lmer’ function in R packages lmerTest (Kuznetsova et al. 2017) and lmtest (Zeileis and Hothorn 2002). We added ‘bird ID’ nested in ‘brood ID’ as random intercept with ‘background position’ (‘in-situ’ or ‘shifted’) as random slope to allow the model compare to the ‘in-situ’ images to the belonging ‘shifted’ images within each individual chick, furthermore “nest ID” as random intercepts. We included ‘year’ as additional random intercept, however, it explained very little of the variance in all models, therefore we omitted ‘year’ from the final models for parsimony. In addition to the random effects, our models contained ‘age’ (‘0 days’ or ‘ > 0 days’) and ‘background position’ (‘in-situ’ or ‘shifted’) as fixed factors. As the hiding behaviour might change when chicks get older and become more mobile, we included the interaction between these two fixed factors and used the ‘emmeans’ function (R package emmeans (Lenth et al. 2023)) for post-hoc comparisons among groups of the main effects.
Digital transfer experiment
To assess the influence of age on camouflage, we examined the differences in luminance, pattern and colour between backgrounds and chicks using LMMs with ‘bird ID’ nested in ‘brood ID’, ‘background ID’ and ‘year’ as random error structure. However, ‘year’ was later removed from the luminance model as it did not explain any of the variance. As fixed effects, we included ‘age’ (‘0 days’ or ‘ > 0 days’) of the swapped chick from the scene 1 picture, ‘background age’ (‘0 days’ or ‘ > 0 days’) of the chick that had chosen the ‘in-situ’ spot from the scene 2 picture, and the interaction between these two fixed factors. As before, we used the ‘emmeans’ function for post-hoc comparisons among groups of the main effects.
Association of chicks with cracks
To evaluate whether chicks were more likely to be associated with cracks than expected by chance, we first tested whether crack overlaps varied between older and younger chicks. We predicted that the ‘in-situ’ place of older chicks would show a higher crack overlap than the hiding place of younger chicks. As the crack distribution differed between images, we compared the observed chick-crack overlap with the distribution of 1000 simulated chick-crack overlaps (see above). For each image, we calculated the Percentile of the observed overlap for each chick within the simulated distribution as follows:
where Obs refers to the observed overlap value between chick and crack, MinSim and MaxSim are the minimum and maximum overlaps between chicks and cracks observed in the simulated data set for each image. We then performed a Mann–Whitney U test to compare whether the observed differences between the age groups were statistically clear. Second, we predicted that chicks would preferably hide in cracks. Here, we tested whether the overlap between cracks and chick shapes was higher than expected by chance. We determined the p-values of the individual chick observed values within their simulated data as following:
used these values to calculate Fisher’s combined probability using the ‘sumlog’ function from the metap R package (Dewey 2020). For all statistical analyses described above, we used R version 4.3.1. (R Development Core Team 2023).
Results
Background matching
Background matching between chicks and their hiding spots varied across different camouflage properties (Fig. 2). After removing the non-significant interaction (Est: -0.077 ± 0.08 [Estimate ± standard error]; t = -0.985; p = 0.329), we observed the strongest match of ‘chick ROI’ and ‘in-situ’ vs ‘shifted’ backgrounds in luminance (Fig. 2a). Luminance differences for ‘chick ROI’ were lowest when measured against their chosen ‘in-situ’ background (Est: 0.106 ± 0.04; t = 6.66; p = 0.003); this match was not clearly affected by chick age (Est: -0.067 ± 0.09; t = -0.75; p = 0.459). In addition, comparing the luminance differences after swapping the ‘in-situ’ backgrounds between older and younger chicks (Fig. 2b) revealed no clear effect of age (background age – Est: -0.086 ± 0.07; t = -1.22; p = 0.230; chick age – Est: 0.042 ± 0.03; t = 1.22; p = 0.231).
Background matching of chicks for pattern, luminance, and colour (i.e., ‘JND’—Just Noticeable Difference). Lower values indicate more optimised background matching. Left panels: chicks in their original background habitat, with their background position either at their chosen hiding spots (‘in-situ’) or at adjacent non-chosen spots (‘shifted’). Individual chicks are represented by data points with connecting lines visualising intra-individual slope differences. Right panels: digitally swapped and transferred chicks on backgrounds of conspecifics, either of the same or different age class. Circles represent raw data points. The boxplots show the median, first and third quartiles (lower and upper hinges) and whiskers show the 95% confidence intervals
For pattern matching, we found an interaction between the main effects: age and background position (Est: 0.306 ± 0.14; t = 2.12; p = 0.038). Older chicks had lower differences between ‘chick ROI’ at the chosen ‘in-situ’ background than at the not chosen ‘shifted’ backgrounds (post-hoc test: p = 0.013) but for younger chicks, there were no clear differences (p = 0.365) (Fig. 2c). A higher suitability of ‘in-situ’ hiding backgrounds chosen by older chicks for crypsis received further support in our digital swap experiment. When we compared pattern matching of chicks to the chosen hiding ‘in-situ’ backgrounds of other chicks (Fig. 2d), we found that, regardless of their own age, chicks had the highest pattern matching at the hiding spots chosen by older chicks (Fig. 2, Est: -0.550 ± 0.21; t = -2.61; p = 0.013) irrespective of the age of the swapped chick (Est: -0.022 ± 0.07; t = -0.34; p = 0.739).
For JND, there was no statistically clear interaction between colour matching for age and background position (age x background position – Est: -0.007 ± 0.05; t = -1.497; p = 0.142; Fig. 2e). After removing the interaction, we found a moderate effect of age suggesting that older chicks showed better colour matching (Est: -0.217 ± 0.02; t = -2.283; p = 0.028) independent of the background they were photographed on (Est: 0.016 ± 0.05; t = 0.796; p = 0.431).
In contrast, for digitally swapped chicks moved to hiding spots of other chicks, we found an interaction between the age of the transferred chick and the age of the chick into whose background it was transferred to (Fig. 2f, age x background age – Est: -0.316 ± 0.14; t = -2.24; p = 0.026). The post hoc analysis showed that this effect was driven by younger chicks, which showed similar colour matching as older chicks for the hiding spots of older chicks (p = 0.511) but a poor match with their own age group’s backgrounds (p = 0.009). Conversely, the colour differences for older chicks were not different for backgrounds chosen by chicks of different age groups (young: p = 0.488, old: p = 0.379).
Association of chicks with cracks
The overlap between the chicks and crack was not clearly affected by age (W = 53, p = 0.824, CI = -43.77 – 44.34). For all chicks with visible cracks on their images, the observed chick overlap with a crack was clearly higher than expected by chance (Fisher’s combined probability: χ2 = 198.47, df = 50, p < 0.001). Only for five of 25 chicks, the percentage of overlap between chick and crack pixels was lower than the 50th percentile of the simulations (Fig. 3). In contrast, for eleven out of 25 chicks, the overlap was above the 95 percentile.
Association of snowy plover chicks with soil cracks is stronger than expected by chance. Top row shows natural variation in cracks with examples of raw, unprocessed photographs of chicks taken in the field. Observed (large circles) and simulated overlap (small dots) between chick and crack pixels for 25 chicks that were photographed on soil patches that contained cracks. Grey (‘non-significant’) box plots indicated that observed value for the chick on a given picture is below 95th percentile for percentage of overlap, orange indicates (‘significant’) that it is above 95th percentile
Discussion
Camouflage is a common strategy of animals to conceal themselves and evade detection. We tested how young precocial chicks employ visual camouflage as an anti-predator defence strategy in their natural environment, in this case an open and sparsely vegetated landscape offering few places to hide. By simulating a sudden predator approach, we tested to what extent young snowy plover chicks were able to choose a hiding spot that optimises background matching based on three variables that are commonly used to describe background matching: luminance, pattern, and colour matching (Troscianko and Stevens 2015). We found partial support for chicks making seemingly adaptive choices and choosing a hiding spot that increased background matching for luminance, pattern and colouration matching. Regardless of age, snowy plover chicks had a higher luminance match with the background at their chosen hiding spots (‘in-situ’ background) than at alternative adjacent spots (‘shifted’ background). For pattern and colouration matching, the efficacy of camouflage was age-related. For pattern matching, we found that older, but not younger chicks were less conspicuous at their chosen hiding spots than at the alternative spots. For colouration matching (JND), we found that older chicks generally had a better colouration match than younger chicks at their chosen hiding spots. In addition, our digital swap experiment suggested that the colouration match of young chicks deteriorated when transferred into the hiding spots of other young chicks, whereas for old chicks, the digital change of their background did not affect the colouration match.
Our study is the first of its kind to assess chick plumage camouflage properties comprehensively. Our results on background matching are in broad agreement with several other studies conducted on camouflage properties of eggs and clutches (Lovell et al. 2013; Stoddard et al. 2016; Troscianko et al. 2016a; Stevens et al. 2017; Gómez et al. 2018). We note, however, that there are also methodological explanations for the observed luminance results. In our study, the high luminance match that chicks showed to the background ROI at their chosen hiding spot did not replicate to adjacent spots or the hiding spots of other chicks after a digital swap of the images of the chick and their background. One potential explanation is that the high match may have resulted from shadows that fell on the chick from surrounding topographical features of the soil, especially when chicks were hiding in cracks. These shadows were then included on the swapped chick but may not have provided an appropriate luminance match at the new spot where the chick was transferred to. Shadows represent a challenge in visual camouflage research (Stevens and Merilaita 2009), although some animals may include cast shadows from other objects to alter their concealment when they are large enough (El Nagar et al. 2021; Kelley et al. 2023). Therefore, it is possible that chicks may use shadows of nearby substrate topography to improve their concealment, however the relevance of shadows for camouflage requires further testing.
Besides luminance matching, we found that pattern matching differed between chosen and not chosen hiding spots. Remarkably, there was an age effect: older chicks had better pattern and colouration matching than younger chicks. This was confirmed in our digital swap experiment. Regardless of the age of the digitally transferred chicks, the hiding spots chosen by older chicks provided the highest pattern and colouration matching for transferred chicks, whereas the corresponding matching was poorer in hiding locations chosen by young chicks. We propose two explanations for the improved concealment at hiding spots by older chicks. First, older chicks may have better cognitive abilities that enables them to choose a better matching hiding spot. However, the age differences between chicks in our test were typically only 2–3 days, suggesting that cognitive differences alone cannot explain the magnitude of the differences we observed. Second, higher mobility may enable older chicks to find a more suitable hiding spot than their younger peers. This is a more plausible explanation than the first, as locomotion abilities of chicks improve quickly after hatching. In snowy plover chicks, tarsi grow approximately linear by as much as 1 mm within the first two days (dos Remedios et al. 2015; Eberhart-Phillips et al. 2020). When following plover families at our study site, we observed that chicks' mobility increases daily, allowing the broods to quickly expand their home ranges with age (CK et al., unpublished data). More mobile chicks can find better hiding spots over increasing distances and/or in a shorter time. In contrast, young and relatively immobile chicks are better off crouching immediately when parents are giving alarm calls, signalling a sudden predator approach. With increasing development and higher mobility, chicks may therefore be able to improve their background matching. Additionally, the plumage of freshly hatched chicks is partly wet and dries within a couple of hours, which might contribute to poorer background matching after hatching. However, camouflage is not expected to increase linearly with chick age. First, larger chicks become also more obvious to predators. Second, when approaching fledging, typically at three to four weeks (Kupán et al. 2021), the mottled downy chick plumage is replaced with juvenile feathers. These ontogenetic changes, which are also reported in other organisms, will therefore impact the crypsis of the chicks and warrant further study (Wilson et al. 2007; Nokelainen et al. 2019).
Although the open landscape of our study system provides limited features for effective hiding, we found that the young chicks seem to make use of what they can find. Chicks associated frequently with soil cracks that emerge on the substrate after the water evaporates: the overlap between chicks and cracks on our pictures was higher than expected by chance. Associating themselves with cracks may provide chicks with several advantages. First, chicks might use the cracks to avoid vertically standing out from the otherwise flat ground. When chicks press themselves into the crack, a predator may be more likely to pass nearby without noticing them than when the chick is lying exposed on the ground. Predators such as reptiles or mammals scanning the ground will have more difficulty finding chicks when they are not exposed on the surface. Second, crouching themselves into a crack will also reduce the shadow that the chick casts. With the strong sunlight that is present at our sub-tropical study site, visual predators will be able to locate 3D objects on the ground based on the shadow that is cast on the soil surface. By hiding in cracks, the chicks will effectively reduce the 3D protrusion of their bodies on the soil and minimise their shadow casting. Third, the presence of cracks may make the detection of chicks harder. This seems counterintuitive at first, as the cracks are usually darker than the chicks, meaning that the background match should be lower. However, the presence of cracks increases background heterogeneity or pattern complexity. Chicks associating themselves with cracks should be harder to spot than those hiding on plain soil. Increased pattern complexity is a feature of camouflage snowy plover adults use to conceal their clutches. Comparing the nests of snowy plovers and least terns Sternula antillarum, which breed sympatrically at our study site, Stoddard et al. (2016) found that the background substrate of snowy plover nests had higher pattern complexity than the substrate of least terns. Fourth, chicks may use the cracks to align their positions to these structures, which may increase their concealment. For example, in Sphingonotus grasshoppers., flight initiation distance is shorter, and detectability is lower for grasshoppers that perch on lines between bricks than those that are not aligned to brick lines in urban habitats (Baños-Villalba et al. 2017). However, exactly how the alignment would make the detection by a predator more difficult remains unclear.
Our study relied on a human vision model as a generalist predator. We acknowledge that the use of other vision models may alter the results on colouration background matching of chicks. Although our field protocols involved taking pictures in the UV light spectrum, we were unable to assess plumage camouflage for UV-reflecting colouration. The required filter change between visible light and UV pictures meant that chicks frequently had changed position, and the required alignment of the photos in the analysis pipeline was not possible. However, for the chicks of our study population, camouflage in the UV spectrum may not be essential. The perception of UV reflection in chick plumage is particularly important for a range of potential avian predators. Yet, chick predation by avian predators is remarkably rare in our study population (MC-L and CK, personal observations). One likely reason for the absence of avian predation is that breeding least terns are very effective in chasing bird predators away. Snowy plover nesting success has been known to increase when the plovers aggregate with breeding least terns (Powell 2001). The protective value may change though. An observed decline in the least tern breeding population because of habitat deterioration over the last years (MC-L et al., unpublished observations) may lead to more avian predation in the future resulting in higher chick (and nest) predation rates.
Camouflage is an important component of defence that is crucial for prey to remain undetected when a predator is in close proximity. However, visual camouflage of chicks is not the only anti-predator strategy that plovers use, and it likely interacts with other anti-predator strategies such as a timely escape, posturing, distraction behaviours or physiological modification such as preen wax volatility to enhance chemical camouflage during incubation in sandpipers (Calidris spp., (Reneerkens et al. 2002)). Previous works have linked escape behaviour to crypsis of incubating birds (Troscianko et al. 2016a) and background matching in grasshoppers (Baños-Villalba et al. 2017). Since the escape abilities of young precocial chicks are not fully developed yet, they have to rely more on their crypsis than older chicks. Posturing behaviour, such as maximising the overlap with soil cracks, seems to be an important hiding strategy of young chicks in an open landscape. Posturing behaviour is also found in other animals during early development. Resting against branches in a posture that makes them appear as twigs conceals larvae of the American pepper moths Biston betularia better from avian predators than larvae resting without the twig-like posture (Rowland et al. 2020). Vigilant snowy plover parents will also contribute to the protection of their young from predators through distraction displays that include injury feigning, such as the broken wing display, predator mobbing or direct attacks (Welling et al. 1997; Sordahl 2004; Griesser and Ekman 2005; Gómez et al. 2018; Humphreys and Ruxton 2020; de Framond et al. 2022). Distraction display varies with the type of predator, distance to the offspring, and the risk that the predator represents for the parents themselves (Sordahl 2004; Gómez-Serrano and López-López 2016; Humphreys and Ruxton 2020). Similar to the closely related Kentish plover Charadrius alexandrinus (Gómez-Serrano and López-López 2016; Gómez et al. 2018), snowy plover parents tending young chicks or hatching clutches often use wing flapping and ‘broken tail’ displays to lure a ground predator away from their vulnerable offspring. The older plover chicks become, the further the parent stays away from the brood and the less intensive these displays become (Colwell et al. 2007). Distraction behaviour by parents might also afford the young chicks some additional moments to find an optimal hiding place. Combining distraction behaviour and cryptic colouration, this synergistic approach should further enhance offspring survival.
Conclusion
Avoiding predation is a major survival challenge for precocial young. The cryptic plumage of plover chicks appears predestined for the study of camouflage in the context of predator–prey interactions (Cott 1940). For young chicks that have limited mobility and cannot easily escape from quickly approaching predators, optimising the camouflage of their plumage features allows them to avoid detection, hence increasing their survival chances during this vulnerable developmental stage. In comparison to the crypsis of eggs and adults (e.g. Nguyen et al. 2007; Colwell et al. 2011; Skrade and Dinsmore 2013; Gómez et al. 2016, 2018; Stoddard et al. 2016; Troscianko et al. 2016a, b; Stevens et al. 2017), yet surprisingly the role of chick plumage for camouflage has received so far little attention. When suddenly forced to hide in an open landscape, young precocial snowy plover chicks seem to preferably choose spots that provide better background matching for luminance. Within a few days after hatching, pattern matching at chosen hiding places improved, thus enhancing the chicks' concealment. Soil features such as cracks were a preferred hiding spot, although it remains to be tested what specific advantages these cracks provide to the chicks. Future studies should assess the fitness consequences of the observed differences in background matching. Such studies should be tailored to the sensory abilities of predator communities (Stuart-Fox et al. 2008; Ekanayake et al. 2015; Galloway et al. 2020).
Data availability
Datasets and original code supporting this article are available on Edmond, the Open Research Data Repository of the Max Planck Society, with the following https://doi.org/10.17617/3.JRKO9Y.
References
Baños-Villalba A, Quevedo DP, Edelaar P (2017) Positioning behavior according to individual color variation improves camouflage in novel habitats. Behav Ecol 29:404–410. https://doi.org/10.1093/beheco/arx181
Camacho C, Sanabria-Fernández A, Baños-Villalba A, Edelaar P (2020) Experimental evidence that matching habitat choice drives local adaptation in a wild population. Proc R Soc B 287:20200721. https://doi.org/10.1098/rspb.2020.0721
Carmona-Isunza MC, Ancona S, Székely T, Ramallo-González AP, Cruz-López M, Serrano-Meneses MA, Küpper C (2017) Adult sex ratio and operational sex ratio exhibit different temporal dynamics in the wild. Behav Ecol 28:523–532. https://doi.org/10.1093/beheco/arw183
Colwell MA, Hurley SJ, Hall JN, Dinsmore SJ (2007) Age-related survival and behavior of snowy plover chicks. Condor 109:638–647. https://doi.org/10.1093/condor/109.3.638
Colwell MA, Meyer JJ, Hardy MA, McAllister SE, Transou AN, Levalley RR, Dinsmore SJ (2011) Western snowy plovers Charadrius alexandrinus nivosus select nesting substrates that enhance egg crypsis and improve nest survival. Ibis 153:303–311. https://doi.org/10.1111/j.1474-919X.2011.01100.x
Cott HB (1940) Adaptive coloration in animals. Methuen & Co., Ltd., London
Cruz-López M, Eberhart-Phillips LJ, Fernández G, Cruz-López M, Eberhart-Phillips LJ, Fernández G, Beamonte-Barrientos R, Székely T, Serrano-Meneses MA, Küpper C (2017) The plight of a plover: viability of an important snowy plover population with flexible brood care in Mexico. Biol Conserv 209:440–448. https://doi.org/10.1016/j.biocon.2017.03.009
Cuthill IC (2019) Camouflage. J Zool 308:75–92. https://doi.org/10.1111/jzo.12682
de Framond L, Brumm H, Thompson WI, Drabing SM, Francis CD (2022) The broken-wing display across birds and the conditions for its evolution. Proc R Soc B 289:20220058. https://doi.org/10.1098/rspb.2022.0058
Dewey M (2020) metap: meta-analysis of significance values. https://CRAN.R-project.org/package=metap. Accessed 06 March 2022
dos Remedios N, Székely T, Küpper C, Lee PL, Kosztolányi A (2015) Ontogenic differences in sexual size dimorphism across four plover populations. Ibis 157:590–600. https://doi.org/10.1111/ibi.12263
Eberhart-Phillips LJ, Cruz-López M, Lozano-Angulo L, Gómez del Ángel S, Rojas-Abreu W, Bucio-Pacheco M, Küpper C (2020) CeutaOPEN, individual-based field observations of breeding snowy plovers Charadrius nivosus. Sci Data 7:149. https://doi.org/10.1038/s41597-020-0490-y
Ekanayake KB, Weston MA, Nimmo DG, Maguire GS, Endler JA, Küpper C (2015) The bright incubate at night: sexual dichromatism and adaptive incubation division in an open-nesting shorebird. Proc R Soc B 282:20143026
El Nagar A, Osorio D, Zylinski S, Sait SM (2021) Visual perception and camouflage response to 3D backgrounds and cast shadows in the European cuttlefish, Sepia officinalis. J Exp Biol 224:jeb238717. https://doi.org/10.1242/jeb.238717
Ferns PN (2003) Plumage colour and pattern in waders. Wader Study Group Bull 100:122–129
Fischler MA, Bolles RC (1981) Random sample consensus: a paradigm for model fitting with applications to image analysis and automated cartography. Commun ACM 24:381–395. https://doi.org/10.1145/358669.358692
Galloway JAM, Green SD, Stevens M, Kelley LA (2020) Finding a signal hidden among noise: how can predators overcome camouflage strategies? Phil Trans R Soc B 375:20190478. https://doi.org/10.1098/rstb.2019.0478
Gómez J-C (2005) Species comparative studies and cognitive development. Trends Cogn Sci 9:118–125. https://doi.org/10.1016/j.tics.2005.01.004
Gómez J, Pereira AI, Pérez-Hurtado A, Castro M, Ramo C, Amat JA (2016) A trade-off between overheating and camouflage on shorebird eggshell colouration. J Avian Biol 47:346–353. https://doi.org/10.1111/jav.00736
Gómez J, Ramo C, Troscianko J, Stevens M, Castro M, Pérez-Hurtado A, Liñán-Cembrano G, Amat JA (2018) Individual egg camouflage is influenced by microhabitat selection and use of nest materials in ground-nesting birds. Behav Ecol Sociobiol 72:142. https://doi.org/10.1007/s00265-018-2558-7
Gómez-Serrano MÁ, López-López P (2016) Deceiving predators: linking distraction behavior with nest survival in a ground-nesting bird. Behav Ecol 28:260–269. https://doi.org/10.1093/beheco/arw157
Green SD, Duarte RC, Kellett E, Alagaratnam N, Stevens M (2019) Colour change and behavioural choice facilitate chameleon prawn camouflage against different seaweed backgrounds. Commun Biol 2:230. https://doi.org/10.1038/s42003-019-0465-8
Griesser M, Ekman J (2005) Nepotistic mobbing behaviour in the Siberian jay, Perisoreus infaustus. Anim Behav 69:345–352. https://doi.org/10.1016/j.anbehav.2004.05.013
Hancock GRA, Grayshon L, Burrell R, Cuthill I, Hoodless A, Troscianko J (2023) Habitat geometry rather than visual acuity limits the visibility of a ground-nesting bird’s clutch to terrestrial predators. Ecol Evol 13:e10471. https://doi.org/10.1002/ece3.10471
Hanlon RT, Chiao CC, Mäthger LM, Barbosa A, Buresch KC, Chubb C (2008) Cephalopod dynamic camouflage: bridging the continuum between background matching and disruptive coloration. Phil Trans R Soc B 364:429–437. https://doi.org/10.1098/rstb.2008.0270
Humphreys RK, Ruxton GD (2020) Avian distraction displays: a review. Ibis 162:1125–1145. https://doi.org/10.1111/ibi.12814
Kang C-K, Moon J-Y, Lee S-I, Jablonski PG (2012) Camouflage through an active choice of a resting spot and body orientation in moths. J Evol Biol 25:1695–1702. https://doi.org/10.1111/j.1420-9101.2012.02557.x
Kelley JL, Jessop A-L, Kelley LA, Troscianko J (2023) The role of pictorial cues and contrast for camouflage. Evol Ecol 37:909–925. https://doi.org/10.1007/s10682-023-10267-z
Kupán K, Székely T, Cruz-López M, Seymour K, Küpper C (2021) Offspring desertion with care? Chick mortality and plastic female desertion in snowy plovers. Behav Ecol 32:428–439. https://doi.org/10.1093/beheco/araa141
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Lenth RV, Bolker B, Buerkner P, Giné-Vázquez I, Herve M, Jung M, Love J, Miguez F, Riebl H, Singmann H (2023) emmeans: estimated marginal means, aka least-squares means. https://CRAN.R-project.org/package=emmeans. Accessed 12 Sept 2023
Lovell PG, Ruxton GD, Langridge KV, Spencer KA (2013) Egg-laying substrate selection for optimal camouflage by quail. Curr Biol 23:260–264. https://doi.org/10.1016/j.cub.2012.12.031
Marshall KLA, Philpot KE, Stevens M (2016) Microhabitat choice in island lizards enhances camouflage against avian predators. Sci Rep 6:19815. https://doi.org/10.1038/srep19815
Mayani-Parás F, Kilner RM, Stoddard MC, Rodríguez C, Drummond H (2015) Behaviorally induced camouflage: a new mechanism of avian egg protection. Am Nat 186:E91–E97. https://doi.org/10.1086/682579
Merilaita S, Scott-Samuel NE, Cuthill IC (2017) How camouflage works. Phil Trans R Soc B 372:20160341. https://doi.org/10.1098/rstb.2016.0341
Nguyen LP, Nol E, Abraham KF (2007) Using digital photographs to evaluate the effectiveness of plover egg crypsis. J Wildlife Manage 71:2084–2089. https://doi.org/10.2193/2006-471
Nokelainen O, Maynes R, Mynott S, Price N, Stevens M (2019) Improved camouflage through ontogenetic colour change confers reduced detection risk in shore crabs. Funct Ecol 33:654–669. https://doi.org/10.1111/1365-2435.13280
Page GW, Stenzel LE, Warriner JS, Warriner JC, Paton PW (2009) Snowy Plover (Charadrius nivosus), version 2.0. In: Poole AF (ed) The Birds of North America. Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bna.154
Plaschke S, Bulla M, Cruz-López M, Gómez del Ángel S, Küpper C (2019) Nest initiation and flooding in response to season and semi-lunar spring tides in a ground-nesting shorebird. Front Zool 16:15. https://doi.org/10.1186/s12983-019-0313-1
Powell AN (2001) Habitat characteristics and nest success of snowy plovers associated with California least tern colonies. Condor 103:785–792. https://doi.org/10.1093/condor/103.4.785
R Development Core Team (2023) R: A language and environment for statistical computing, version 4.3.1. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org. Accessed 10 Sept 2023
Reneerkens J, Piersma T, Sinninghe Damsté JS (2002) Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proc R Soc B 269:2135–2139. https://doi.org/10.1098/rspb.2002.2132
Rohr VA, Volkmer T, Metzler D, Küpper C (2021) Neoptile feathers contribute to outline concealment of precocial chicks. Sci Rep 11:5483. https://doi.org/10.1038/s41598-021-84227-4
Van Rossum G, Drake FL (2009) Python 3 Reference Manual. CreateSpace, Scotts Valley, CA
Rowland HM, Burriss RP, Skelhorn J (2020) The antipredator benefits of postural camouflage in peppered moth caterpillars. Sci Rep 10:21654. https://doi.org/10.1038/s41598-020-78686-4
Rublee E, Rabaud V, Konolige K, Bradski G (2011) ORB: An efficient alternative to SIFT or SURF. 2011 International Conference on Computer Vision. IEEE, Barcelona, pp 2564–2571
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Skelhorn J, Rowe C (2016) Cognition and the evolution of camouflage. Proc R Soc B 283:20152890. https://doi.org/10.1098/rspb.2015.2890
Skrade PDB, Dinsmore SJ (2013) Egg crypsis in a ground-nesting shorebird influences nest survival. Ecosphere 4:art151. https://doi.org/10.1890/ES13-00246.1
Sordahl TA (2004) Field evidence of predator discrimination abilities in American avocets and black-necked stilts. J Field Ornithol 75:376–385
Stevens M, Merilaita S (2009) Animal camouflage: current issues and new perspectives. Phil Trans R Soc B 364:423–427. https://doi.org/10.1098/rstb.2008.0217
Stevens M, Ruxton GD (2019) The key role of behaviour in animal camouflage. Biol Rev 94:116–134. https://doi.org/10.1111/brv.12438
Stevens M, Troscianko J, Wilson-Aggarwal JK, Spottiswoode CN (2017) Improvement of individual camouflage through background choice in ground-nesting birds. Nat Ecol Evol 1:1325–1333. https://doi.org/10.1038/s41559-017-0256-x
Stockman A, Sharpe LT (2000) The spectral sensitivities of the middle- and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vision Res 40:1711–1737. https://doi.org/10.1016/S0042-6989(00)00021-3
Stoddard MC, Kupán K, Eyster HN, Rojas-Abreu W, Cruz-López M, Serrano-Meneses MA, Küpper C (2016) Camouflage and clutch survival in plovers and terns. Sci Rep 6:32059. https://doi.org/10.1038/srep32059
Stuart-Fox D, Moussalli A, Whiting MJ (2008) Predator-specific camouflage in chameleons. Biol Lett 4:326–329. https://doi.org/10.1098/rsbl.2008.0173
Székely T, Kosztolányi A, Küpper C (2008) Practical guide for investigating breeding ecology of Kentish plover (Charadrius alexandrinus). Unpublished Report, University of Bath, Bath
Troscianko J, Stevens M (2015) Image calibration and analysis toolbox – a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol Evol 6:1320–1331. https://doi.org/10.1111/2041-210X.12439
Troscianko J, Wilson-Aggarwal J, Spottiswoode CN, Stevens M (2016a) Nest covering in plovers: how modifying the visual environment influences egg camouflage. Ecol Evol 6:7536–7545. https://doi.org/10.1002/ece3.2494
Troscianko J, Wilson-Aggarwal J, Stevens M, Spottiswoode CN (2016b) Camouflage predicts survival in ground-nesting birds. Sci Rep 6:19966. https://doi.org/10.1038/srep19966
Troscianko J, Skelhorn J, Stevens M (2017) Quantifying camouflage: how to predict detectability from appearance. BMC Evol Biol 17:7. https://doi.org/10.1186/s12862-016-0854-2
Twort L, Stevens M (2023) Active background selection facilitates camouflage in shore crabs, Carcinus maenas. Anim Behav 203:1–9. https://doi.org/10.1016/j.anbehav.2023.06.007
Welling PP, Rytkonen SO, Koivula KT, Orell MI (1997) Song rate correlates with paternal care and survival in willow tits: advertisement of male quality? Behaviour 134:891–904. https://doi.org/10.1163/156853997X00214
Wilson D, Heinsohn R, Endler JA (2007) The adaptive significance of ontogenetic colour change in a tropical python. Biol Lett 3:40–43. https://doi.org/10.1098/rsbl.2006.0574
Xiao F, Cuthill IC (2016) Background complexity and the detectability of camouflaged targets by birds and humans. Proc R Soc B 283:20161527. https://doi.org/10.1098/rspb.2016.1527
Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2:7–10
Acknowledgements
We thank Ivan Guardado-González for logistic support during fieldwork and Mary C. Stoddard for early discussion of the study design. We also thank Nils Linek for discussion of the methods and results. We are grateful for the constructive comments of two anonymous reviewers that helped to improve earlier versions of this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the Max Planck Society (to CK). Field work expenses by TV were covered by the Office of International Relations and the Naturwissenschaftliche Fakultät of the University of Graz. Field work expenses for SGdelA were provided by the Postgraduate Program of the Institute for Marine Sciences and Limnology, Universidad Nacional Autónoma de México.
Author information
Authors and Affiliations
Contributions
TV: conceptualisation, funding acquisition, data collection, data analyses, interpretation of results, writing – original draft, writing – editing; KK: data analyses, interpretation of results, writing – original draft, writing – editing; VAR-B: data analyses, interpretation of results, writing – editing; MG-O: data analyses, interpretation of results, writing – editing; MC-L: permit acquisition, data collection, writing – editing; SGdelA: data collection, writing – editing; LF-R: data collection, writing – editing; LEH: data collection, data analyses, interpretation of results, writing – editing; CK: conceptualisation, supervision, funding acquisition, data collection, data analyses, interpretation of results, writing – original draft, writing – editing.
Corresponding author
Ethics declarations
Ethics approval
Fieldwork permits to handle and mark birds were provided by Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), permit number SGPA/DGVS/009795/18. All applicable international, national, and/or institutional guidelines for the use of animals were followed. No animals were harmed during this study and no further ethics approval was required.
Competing interests
The authors declare that they have no competing interests.
Additional information
Communicated by Kevin McGraw.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Volkmer, T., Kupán, K., Rohr-Bender, V.A. et al. Hidden in plain sight: camouflage and hiding behaviour of wild precocial chicks in an open landscape. Behav Ecol Sociobiol 78, 73 (2024). https://doi.org/10.1007/s00265-024-03482-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03482-3