Abstract
Fission–fusion dynamics describe the tendency for members of some animal societies to associate in subgroups that change size and structure fluidly over time. These dynamics shape social complexity and social structure, but are difficult to study because they unfold simultaneously over large spatial scales. Here we use simultaneous, fine-scale GPS data from spotted hyenas to examine fission–fusion dynamics through a dyadic analysis of merge-split events between pairs of individuals. We introduce a species-agnostic framework for identifying merge-split events and discretizing them into three phases (merging, together, and splitting), enabling analysis of each phase as well as the connections among phases. Applying this framework to the hyena data, we examine the temporal and spatial properties of merges and splits between dyads and test the extent to which social encounters are driven by key locations. Specifically, we focus on communal dens—shelters for juvenile hyenas where classical observational studies often report large aggregations of adults. We find that overall, 62% of merges occurred at communal dens, supporting the idea that dens facilitate meet-ups and subsequent social behavior. Social encounters most commonly involved close approaches within a few meters between hyenas, while co-travel together occurred in only 11% of events. Comparison to permutation-based reference models suggests that independent movement decisions structure broad-scale patterns of social encounters but do not explain the fine-scale dynamics of interactions that unfold during these encounters. We reflect on how physical features such as dens can become social hotspots, causing social and spatial processes to become fundamentally intertwined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In social groups, the spatial arrangements and social interactions among individuals are highly dynamic, changing fluidly as individuals go about their daily lives (Webber et al. 2023). Although all groups exhibit these changing social landscapes, they are particularly dramatic in species with high degrees of fission–fusion dynamics (Aureli et al. 2008). In these species, individuals associate in subgroups that change in size and composition frequently, as individuals or subgroups merge (fusion) and split (fission) over time. By shaping which individuals are available as social partners, subgroup fissions and fusions structure dyadic encounter rates and form the fundamental building blocks of social structure in these societies. Social systems with a high degree of fission–fusion dynamics are taxonomically diverse, occurring in many birds (Silk et al. 2014), fish (Kelley et al. 2011), and mammals (Mann et al. 2000; Archie et al. 2006), including human societies (Marlowe 2005).
Because of the fluidity of these systems, individuals gain some control over their social environment through choice of association partners. Many societies that have a high degree of fission–fusion dynamics also show a high degree of social complexity, as measured by the degree of differentiation in social relationships or uncertainty in the social environment an individual experiences over time (Lukas and Clutton-Brock 2018; Ramos-Fernandez et al. 2018). Individuals experiencing conflicts of interest with their subgroup partners can leave the subgroup and join a new one, thus relaxing the need to form consensus among all individuals and consequently reducing consensus costs (Conradt and Roper 2005). However, this increased choice of with whom to associate also presents challenges to individuals. Whereas social partners are close-at-hand in more cohesive groups, individuals living in fission–fusion groups can less reliably find social partners, especially if they prefer socializing with particular group-mates. Existing work has focused primarily on the socioecological factors influencing subgrouping patterns (Chapman et al. 1995; Silk et al. 2014), but less is known about where fusion events occur and how spatial components of reunions influence subsequent social cohesion.
Fusion events occur when two different individuals or subgroups encounter one another, but these encounters can occur in multiple ways and lead to varying post-fusion social behavior. Encounters between individuals can be facilitated by key locations—for instance at sleeping sites like dens or roosts, or clumped resources such as fruit trees or watering holes. These spatial attractors can serve as catalysts for social encounters, thus influencing social structure (Mourier et al. 2012; Farine et al. 2015; Firth and Sheldon 2015). When key locations remain fixed over time, they can be reliable places to engage in fusions. Individuals or subgroups may visit these key locations through independent movement decisions or through coordinated travel to and from the locations, and permutation-based reference models are a useful tool for distinguishing the extent to which grouping patterns are produced by independent vs. coordinated movement decisions (Spiegel et al. 2016; Hobson et al. 2021). In addition to being driven by key locations, fusions can occur when individuals spontaneously encounter one another away from key locations—here again, the encounters could be due purely to independent movement decisions of each individual or subgroup, or through active seeking of spatial proximity with group-mates.
Little is known about how these different processes shaping fusions might influence post-fusion sociospatial behavior. For instance, fusions that occur at or away from key locations may differ in their levels of coordination, duration, or frequency of social interaction. Post-fusion social behavior might also be influenced by the activities of the two parties immediately prior to fusion (e.g., if one is resting and another is traveling). Furthermore, in many species, it is unclear whether key locations are hotspots of social activity because individuals meet up there, or because individuals meet elsewhere to travel to these locations. For instance, in tree roosting bats, grouping is typically monitored at roosts (Wilkinson et al. 2019), so less is known about how frequently fusions occur outside of roosts or how individuals come to occupy the same roosts (but see Ripperger and Carter 2021; O’Mara and Dechmann 2023). Limitations of on-the-ground field observations—for instance the difficulties of following multiple individuals simultaneously, at all hours of the day, and in all terrains—make it challenging to assess in an unbiased way where, when, and how often fusions occur. To address this challenge, it is necessary to have (a) a system for monitoring the movements of multiple group members at once, and (b) an analytical approach for defining and quantifying fission–fusion dynamics.
In this study, we develop a system for studying the spatial properties of fusion events between pairs of individuals (hereafter referred to as “merges”) and their consequences for post-merge behavior in spotted hyenas (Crocuta crocuta), an ideal system in which to explore these questions. Spotted hyenas (henceforth, hyenas) are carnivores living in closed groups, called clans, that defend a common territory and show a high degree of fission–fusion dynamics within each clan (Kruuk 1972; Aureli et al. 2008), with individuals joining together and meeting up multiple times per day (Smith et al. 2008). Hyena clans are composed of multiple matrilines, with high relatedness within matrilines but low average relatedness within the group (Van Horn et al. 2004). Although clans can contain over 100 individuals (Green et al. 2018), hyenas associate in much smaller subgroups, with roughly 60% of subgroups composed of only one or two individuals (Smith et al. 2008). Fission–fusion dynamics help individuals reduce the costs, while retaining the benefits, of sociality. Spending time alone or in small subgroups reduces feeding competition (Holekamp et al. 1997b; Smith et al. 2008), and may also protect young offspring from infanticide (Smith et al. 2008; Brown et al. 2021). However, hyenas form larger subgroups to engage in cooperative behavior such as interclan conflict, competition with sympatric carnivores, or patrolling territorial boundaries (Smith et al. 2008; Lehmann et al. 2017, Montgomery et al. 2023). It is not only subgroup size that is relevant for spotted hyenas, but also which individuals are present in the subgroup. Spotted hyenas exhibit differentiated social relationships that are correlated with tolerance during feeding competition (Smith et al. 2007) and variation in social support, which is linked to dominance rank and fitness (Strauss and Holekamp 2019). Therefore, the challenge of finding and interacting with social partners is critical in this species.
The three behavioral processes facilitating the meet-ups discussed above—chance encounters, long distance communication, or key locations—may all operate to varying degrees in hyenas. Hyenas emit long-distance calls that can facilitate convergence with other individuals (Gersick et al. 2015), as in bonobos (Schamberg et al. 2017). Additionally, hyena communal dens are spatiotemporally stable key locations that might allow individuals to locate and associate with preferred partners, and large subgroups of hyenas are often found there. Communal dens are large complexes where females keep their young offspring until they are 10–12 months old (Mills 1990; Holekamp and Smale 1998). Mothers with dependent offspring visit the den daily to nurse, and though hyenas do not engage in allocare, individuals without young offspring nevertheless visit the den regularly, presumably for social reasons (Holekamp and Smale 1998). Although dens are known to be socially important for hyenas, the extent to which they drive aggregate fission–fusion dynamics and social interaction patterns remains unknown.
Here we use multi-sensor collars to track the movements of multiple hyenas within the same clan simultaneously. High-resolution GPS collars are increasingly useful tools for studying the dynamics of social behavior in a variety of species (He et al. 2022). We develop a general framework for modeling dyadic “merge-split events” (i.e. splits and merges between pairs of individuals) as consisting of three canonical phases (merge, together, and split), distinguished by changes in distance between the two individuals over time. We refer to these dyadic level events as merge-split events throughout, to distinguish them from more broad-scale patterns of fission–fusion dynamics involving subgroups of individuals. This framework is powerful in that it can be applied to any system with GPS data on movements of animals, and can be used to analyze the properties of each phase as well as the connections among the phases. Using this approach, we quantify the properties of merges and splits, explore how spatial properties of a merge influence post-merging behavior, and assess the role of communal dens as drivers of hyena social encounters. Finally, we analyze the extent to which features of observed dyadic merge-split events and social networks constructed from these events differ from expectations under a reference model where individuals move independently of one another.
Methods
Data collection
We used custom-built tracking collars to collect data on the movements of five wild adult female spotted hyenas belonging to the same clan in the Masai Mara National Reserve, Kenya. Collars were deployed throughout December 2016, and recorded continuously from January 1 until mid-February 2017 (Table S1, Supplementary Video 1). Although these were few individuals, our analyses were not aimed at explaining individual differences in behavior or dyadic differences in social relationships. Instead, we use the data from these individuals to understand the spatial distribution and mechanics of merge-split events (see Data analysis), and we observed many of these events (n = 551). Similarly, this sampling window is too short to capture variation in social behavior in response to seasonal changes in prey availability, but prior work in this system has demonstrated that high degrees of fission–fusion dynamics are consistent across environmental conditions (Holekamp et al. 2012).
These hyenas were observed as part of the Mara Hyena Project, a long-term study of several hyena clans ongoing since 1988. Individuals in this study were monitored near-daily from birth, providing important information about genealogical (Holekamp et al. 2012) and dominance relationships among members of the group (Strauss and Holekamp 2019). Daily monitoring data were used to identify the locations of communal dens, and subjects used four different communal dens over the course of the study (Fig. 1C, S2). Data collection was blind to individual identity because data were collected remotely using GPS collars. To obtain a representative sample of individuals, we collared females who weren’t closely related or closely positioned in the dominance hierarchy. We also selected females with variable reproductive states—two had den-dependent cubs throughout the study, one gave birth halfway through the study, one was between reproductive events, and one had a den-independent but unweaned cub. We elected to sample individuals with diverse reproductive states and ranks because both of these variables are known to influence individual movement decisions (Holekamp and Strauss 2020). However, because dominance rank similarity is associated both with kinship relationships and social bond strength (Holekamp et al. 2012), this approach led us to sample dyads that were not among the most strongly bonded in the group.
Spatial and temporal patterns of social encounters in spotted hyenas. A Female hyena wearing a tracking collar. B Time of day and C locations of the starts of merge-split events (i.e. merges) across all hyena pairs. Color specifies whether events started at a den (blue) or not (magenta). White circles represent locations of the four communal dens in use during the study period. See also Supplementary Video 1
Each hyena wore a Tellus Medium collar (Followit Sweden AB) containing a custom-built sound and movement module modified from a DTAG board (Johnson and Tyack 2003; Johnson et al. 2009) and integrating a high-resolution (95% of points within < 5 m), high sample rate GPS (Gipsy-5 module, Technosmart, Italy). We focus primarily on the GPS (1 Hz) data in this study, and also make use of the triaxial accelerometer (1000 Hz, down-sampled to 25 Hz) data for quantifying activity similarity. Prior to analysis, we performed minimal pre-processing of the GPS data to remove unrealistic locations and fill in very short gaps. The GPS data also contained some 12-h gaps due to a firmware bug – such missing data accounted for 18% of the total tracking time. See Supplementary Material 1 and 2 for details on collar specifications, collar deployment, data preprocessing, and missing data.
Data analysis
We used the GPS data to identify merge-split events involving each pair of hyenas, and mapped the spatial and temporal distribution of these events, specifically in relation to dens. We then characterized the dynamics of these events in two ways. First, we developed a framework for breaking each event into discrete phases, then categorized and combined these phases to produce a “taxonomy” of event types. Second, we quantified the properties of events via several continuous metrics (Table S2) and analyzed the distribution of these metrics for events occurring both at and away from dens. Next, we tested how well reference models that accounted for independent movement decisions about den usage and daily ranging could capture the typical properties of events, as well as aggregate interaction patterns. Lastly, we constructed a social network based on the frequency of merge-split events for pairs of individuals and compared this network to networks produced by our reference models.
Identifying dyadic merge-split events
To identify merge-split events involving pairs of individuals, we considered the perspective of an individual moving throughout the landscape and repeatedly encountering other animals. Each of these encounters can be viewed as a sequence of three distinct phases: the two individuals come together (merging phase), spend some amount of time in association (together phase), then eventually separate (splitting phase). Following from this simple model, we define a “merge-split event” as a sequence of these three phases. We identified the occurrence of a merge-split event as any time two individuals came within 100 m of one another, then considered the event to start when they first got within 200 m and end when they separated by at least 200 m. We did not impose any temporal constraints on the definition of what constitutes an event (e.g. a minimum event duration). Using two distance thresholds rather than a single one avoided the problem of introducing many short “events” when individuals crossed a single threshold multiple times due to noise or small movements. We chose the values of the distance thresholds to be consistent with long-standing definitions used by Mara Hyena Project personnel during direct behavioral observations in the field, where individuals are considered together when within 200 m of one another (Holekamp et al. 1997a). We also conducted two additional analyses to assess the validity of this threshold: first, we examined distributions of dyadic distances and how they relate to measures of coordination to identify spatial scales at which these quantities show transitions (Fig. S1). Second, we conducted a sensitivity analysis by rerunning all analyses using alternative threshold values to confirm that the choice of threshold values did not qualitatively change the results (Supplementary Material 6).
Analyzing the dynamical properties of merge-split events
The dynamics observed during merge-split events vary, yet all events share common features. The distance between the two individuals by definition follows a U-shaped structure during a merge-split event (Fig. 2B), declining as they converge (merging phase), remaining small while they are associated (together phase), then rising again as they part ways (splitting phase). We took advantage of this canonical structure to identify the three phases for each event by fitting a U-shaped function constructed of three line segments (Fig. 2B) using constrained piecewise linear regression with least squares minimization (Supplementary Material 1). We constrained the three segments such that the first started at 200 m, the last ended at 200 m, and the middle segment had a slope of 0. Note that the “height” of the middle segment is a fitting parameter in the model and is not constrained to be any particular value (because the hyenas’ average inter-individual difference while together varies across events). As an output of this fitting procedure, we identified transition times (b1 and b2) which allowed us to decompose each event into the merging, together, and splitting phases.
Example merge-split event and frequencies of event types. A Example trajectories of two individuals during an extracted merge-split event. Gray lines connect time-matched GPS points during the together phase; S and E denote start and end of trajectories, respectively. See Supplementary Videos for animated examples. B Distance between the two individuals over time (black) and fitted piecewise regression model (red). Break points (b1 and b2) are used to identify the three phases for each event. C Alluvial plot of the frequencies of transition motifs between different categories of the three phases. Symbols indicate the movement patterns of the two individuals involved in the event (• = stationary, ↑ = moving, ⊕ = local, ⇑ = traveling). Note that asymmetrical splits can occur in two ways: either the two individuals show the same movement patterns as in the merge phase (•↑), or the individuals reverse movement patterns (↑•). Flow bars are colored by the type of merge to facilitate identifying effects of merge type on post-merge behavior. For instance, most merge events involving both individuals moving lead to stationary together events (top two grey bars coming out of Merge ↑↑)
To understand how properties of merges relate to subsequent behavior, we used movement patterns of the individuals in each phase to build a “taxonomy” of event types. We classified the merging phase into two phase categories—both individuals moved (↑↑), or one was stationary while the other moved (•↑). An individual was classified as having “moved” if its displacement between the beginning and end of the phase was greater than 5 m, an upper bound on our estimated GPS error. The splitting phase had three potential categories: both moving (↑↑), one stationary and one moving in the same arrangement as the merging phase (•↑), or one moving and one stationary but with the movement roles reversed (↑•). Note that it was not possible for both individuals to remain stationary during these phases, as movement of at least one individual is necessary to result in a merge or split. We classified the together phase into “traveling” (⇑) if the individuals had a displacement of greater than 200 m during the phase, or “local” ( ⊕) if not.
Using these phase category definitions, we then analyzed the typical sequences of phase categories seen in our data (Fig. 2C). Combining the phase categories allowed us to classify each event into one of ten distinct event types (Fig. 3B).
Comparison of the number of merge-split events in real data vs. reference models preserving den attendance and daily ranging patterns. A Overall number of events observed across real data and reference models across all events, den events, and non-den events. Lines represent values observed in the real data, and violin plots represent the distribution of values in reference models (100 instantiations). B Frequency of events (x-axis) broken down by type (y-axis) in the real data (vertical lines) as compared to the reference models (violin plots). Y-axis labels represent the behavior of the two individuals during the three phases (from left to right: merge, together, split) for each event type. Symbols indicate the movement patterns of the two individuals involved in the event (• = stationary, ↑ = moving, ⊕ = local, ⇑ = traveling). Dotted lines connect pairs of event types that are essentially time-reversed versions of each other, to highlight the asymmetry between merges and splits (see text)
For example, one possible event type is the category in which “one individual approaches another and then leaves” (Supplementary Video 2):
-
1)
Merging phase – one individual is stationary, one is moving: “•↑”
-
2)
Together phase – local: “ ⊕ ”
-
3)
Splitting phase – the stationary individual remained stationary, the moving individual continued moving: “•↑”
We represent the complete event-type graphically as “•↑—⊕—•↑.”
Alternatively, the event type •↑—⇑—↑↑ represents one individual approaching another that was stationary, then the two moving off and traveling together before mutually parting ways (Supplementary Video 3). After classifying events into types, we analyzed how often each event type occurred in our data to assess what types of merge-split events are characteristic of hyena interactions.
We also characterized the properties of events through continuous metrics: event duration; displacement, directional and activity synchrony during the together phase; and distance from the den at the start and end of the event (Table S2).
Temporal and spatial distribution of merge-split events, and their relationship to dens
To characterize where merge-split events typically occur, we classified merge-split events according to their spatial proximity to dens (Fig. 1C), defining “den events” as events that either started or ended within 200 m of a communal den, with the remaining events considered “non-den events”.
Constructing association networks based on merge-split events
To quantify broader patterns of social structure amongst our tracked hyenas, we constructed a social network. We defined edge weights using a version of the simple ratio index (Farine and Whitehead 2015):
where nij represents the number of merge-split events (i.e. number of events where they were together, or associations) involving individuals i and j, ni the number of events involving individual i, and likewise for nj. This metric quantifies the extent to which i and j associate with one another as a fraction of their associations with all other tracked individuals.
Permutation-based reference models for merge-split events
To test to what extent independent den usage and daily ranging patterns underlie the observed properties of merge-split events, we constructed permutation-based reference models. To do so, we permuted our data such that the trajectory of each individual for a given day was randomly assigned to a different day (Spiegel et al. 2016). This permutation preserves each individual’s overall ranging patterns and typical daily patterns of movement, including any habitual use of certain locations or routes at specific times of day, but breaks the temporal link between the trajectories of pairs of individuals. Because communal den locations changed during the study, we accounted for den usage by constraining the permutation to only swap days from periods where the individual was using the same den or set of dens (Fig. S2). We also constrained the permutations such that no two individuals were randomly “matched” to the same day, thus ensuring a complete break-up of the temporal links between trajectories. To minimize possible artefacts arising from temporal discontinuities at the “break point” between days, we used noon as the break point (because hyenas are generally least active around mid-day) and also removed any events crossing noon in both observed data and reference models from all analyses involving the reference models.
For each reference model (n = 100 permutations), we carried out the same analyses as described above (i.e. extracting events, characterizing their phases and types, computing their properties, and constructing a social network) to allow comparison with the real data.
Results
When and where do merge-split events occur?
Overall, we identified 690 merge-split events involving the five tracked hyenas, for 551 of which we could identify the exact start and end times enabling further analysis (Supplementary Material 1). Events were more likely to occur at night than during the day, with peak occurrence around dusk and dawn (Fig. 1B). Median duration of the together phase of events was 20.58 (IQR = 3.23—68.48) minutes, and dyads engaged in a mean of 2.22 (range = 0.68 – 3.60 across dyads) merge-split events per day of simultaneous observation time. Analyzing the spatial distribution of these events (Fig. 1C) revealed that 62% of merges (n = 339 events) and 57% of splits (n = 315 events) occurred at a communal den, with a total of 64% of all events either starting or ending at a den (n = 350 events).
Using classical daily observation-based sampling (see (Holekamp et al. 2012) for details) over the same time period, we observed pairs of our tagged individuals in 39 encounters (33 occurring at dens, 6 away from dens). Additionally, we observed our five tagged individuals interact with a total of 87 other clan-mates (of all ages and sexes). This comparison reveals how our sampling approach is poorly suited for capturing the different types of individuals that were engaging in social events, but provides an unprecedented view into the timing, frequency, location, and mechanics of merge-split events that is unbiased by factors constraining classic observational studies.
It is important to note that we here take a dyadic perspective on fission–fusion dynamics, whereas in reality, many encounters in hyena societies involve more than two participants. We explore such polyadic events in Supplementary Materials Section “Constructing association networks based on merge-split events”, though we note that a full investigation is beyond the scope of the current work due to the limited number of hyenas in our sample.
What types of merge-split events are observed?
Our phase categorization scheme (Fig. 2C) revealed that for most events (89%), the together phase was local ( ⊕), i.e. the two individuals did not travel more than 200 m while together. For events where the together phase involved travel (⇑), these were more likely to result from merges where both individuals were initially moving and their paths converged (↑↑; 60% of traveling events) than by merges where one individual moved to meet a stationary hyena (•↑, 40% of traveling events). Traveling events most often ended with both individuals splitting by continuing to move while their paths diverged (↑↑), rather than with a single individual remaining stationary while the other moved off (•↑ or ↑•). Of events involving joint travel, 36% started at the den and 9% ended at the den, indicating that individuals more often met up at the den and traveled elsewhere than traveled together to the den.
Although the definition of merge-split events is symmetric, such that one could think of a split as simply a merge in reverse, our data revealed an asymmetry in how splits vs. merges occur (Fig. 3B; compare events connected by dashed lines). It was more common for individuals to engage in an interaction where one was initially stationary in the merging phase and later both moved off during the splitting phase than the reverse. In other words, it was more common for individuals to meet by arriving in sequence to a given location and then move off at the same time than it was for them to arrive synchronously and leave asynchronously.
What are the properties of typical merge-split events?
Our method for identifying merge-split events and breaking them into three phases allowed us to characterize typical properties of these events, and to compare post-merging behavior during den and non-den events (Fig. 4). Overall, merge-split events showed a wide range of durations (Fig. 4A), with the duration of events where merging occurred at the den (median = 44 min, IQR = 14 – 101 min) typically longer than those where merging occurred away from the den (median = 14 min, IQR = 7 – 34 min). Regardless of where merging events occurred, hyenas tended to come into close proximity (Fig. 4C), with 76% and 74% of den and non-den events respectively involving approaches to within 3 m. Extended travel together was relatively rare (Fig. 4B), and hyenas tended to travel longer distances together when merging occurred away from the den (median = 23 m, IQR = 2 – 118 m) than at the den (median = 16 m, IQR = 4 – 42 m). Overall, 13% of den events and 28% of non-den events involved co-travel for more than 200 m. Finally, while hyenas showed high levels of heading similarity (Fig. 4E) during both den (76% events > 0 similarity, 50% events > 0.5 similarity) and non-den (77% events > 0 similarity, 57% events > 0.5 similarity) events, they showed higher levels of activity similarity (Fig. 4F) during non-den events (75% events > 0 similarity, 19% events > 0.5 similarity) than den events (67% events > 0 similarity, 5% events > 0.5 similarity).
Reference models reproduce some but not all properties of observed merge-split events. Detailed properties of merge-split events in the real data (thick lines) and in reference models (thin lines), broken up by whether the event occurred in the vicinity of a den (blue) or not (magenta). Plots show the cumulative distribution of each metric (x-axis labels) across all events, with the exception of panel D, which shows the distribution rather than the cumulative distribution. Three types of insight can be gained from these plots: 1) interpretation of the distribution of observed properties. For instance, although merge-split events were defined on a scale of 100-200 m, three quarters of events involve a close approach to within 3 m (C). 2) Comparison of den and non-den events. For instance, den and non-den events show remarkable differences in a number of spatial properties (A, B, F, G, H). 3) Comparison of observed properties with those from reference models. For instance, reference models captured properties of events occurring at dens better than those occurring away from dens (A—D)
To what extent are merging and splitting patterns explained by independent daily ranging and den usage?
Our permutation-based reference models revealed that a majority of the observed number of merge-split events would be expected purely based on independent daily ranging and den usage (Fig. 3A). In particular, the reference models predicted a median of 379 events (95% range: 349—418), which is 70% of the number of observed events (543). When considering the locations of merges, the reference models predicted a median of 310 merge events at dens (95% range: 289—340), compared to 339 den events in the real data. Thus, the reference models accounted for approximately 90% of den events. In contrast, the reference models predicted a median of 66 (95% range: 52–85) events occurring away from dens, which is only 32% of those observed (204 non-den events).
There was variation in how well the reference models captured different types of events (Fig. 3B). While most event types were underrepresented in the reference models compared to the real data, the number of local events where one of the individuals remained stationary during both the merging and the splitting phase (•↑—⊕—•↑, Supplementary Video 2) was actually slightly overrepresented. Conversely, events involving both individuals moving off during the splitting phase were particularly underrepresented. Despite the overall lower number of events in the reference model, the relative frequencies of different event types were similar between the observed data and the reference model.
When quantifying continuous properties of merge-split events (Table S2), the reference models captured some properties much better than others (Fig. 4). Specifically, the distribution of event durations was approximately the same in the reference models as in the real data (Fig. 4A), as was the distribution of events across the day (Fig. 4D). However, distances traveled by dyads during the together phase were in general greater during real events than during artificial events generated by the reference models (Fig. 4B), and hyenas approached each other much more closely in the real data (Fig. 4C). Hyenas also had higher heading similarity (Fig. 4E) and activity synchrony (Fig. 4F) during real events, although activity synchrony at the den was reasonably well captured by the reference models.
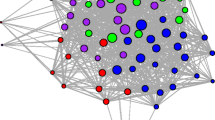
Scaling up from individual events to social networks, we found that the reference models accurately captured the observed patterns of association amongst the five tagged individuals (Fig. 5). Because of the few individuals monitored in this study, we do not view this network as representative of the network structure of the group, nor do we directly interpret the values of the network edges. Instead, we evaluate how well networks generated from reference models match the observed data. Empirically measured edge weights in the association network all fell within the expected ranges of values from the reference models, and the overall relative ranking of edge weights across dyads in the reference models was consistent with that observed in the real data. These results indicate that accounting for independent daily ranging and den usage patterns alone was enough to broadly explain the association patterns of the spotted hyenas observed in this study.
Reference models reproduce differentiated relationships found in observed social networks built from merge-split events. [Top panels] Observed association network as well as three example networks built from merge-split events in the permutation-based reference model. Note the close similarity between networks from the reference model and the observed network. [Bottom panel] Black lines indicate observed edge weights representing frequency of association and corresponding to the ties in the observed network in the top panel. Violins depict distributions of edge weights in 100 instantiations of the reference model (i.e., distribution of ties from reference model networks in top panel). Black lines fall within the distributions of tie strengths from the reference model, indicating that observed edge weights are not significantly different from those found in the networks built from reference models
In sum, the results from the reference model reveal how certain features of observed merge-split events—namely, event duration and partner identity—can arise from independent movement decisions of individuals, whereas others— including synchrony, close proximity, and co-travel—can only be explained by accounting for other processes (e.g., coordination).
Discussion
We present here a framework for measuring dyadic merge-split events and use it to characterize the properties of these events, the role of communal dens as key locations fostering social encounters, and the extent to which independent decisions about daily ranging patterns underlie social structure in spotted hyenas. We found that over 60% of merge-split events in our study either began or ended at a den, quantifying the critical role as social hub played by communal dens for hyenas. Linking merges to post-merging behavior clarified how communal dens serve as social hubs; of all merging events involving joint travel, individuals were four times as likely to meet up at the den and travel elsewhere together than they were to meet elsewhere and travel to the den, suggesting that the communal den acts more as a facilitator of encounters between hyenas than a destination for socializing dyads. Interactions occurring away from dens showed key differences from those occurring at dens, including shorter durations, more co-travel, and a greater level of coordination in headings and activity states, and the two types of events also showed peaks of occurrence during different hours of the day (Fig. 4D). These differences suggest that social interactions at and away from dens may reflect different components of hyena sociality and may thus be important to disambiguate when studying hyena social structure.
Reference models capturing events that would arise from independent decisions about daily movement patterns and den attendance were sufficient to explain many, but not all, features of the observed patterns of merge-split dynamics in hyena societies. Reference models effectively captured the duration of merge-split events, the time of day at which they occurred, and the distribution of identities of merging partners, but underestimated the overall frequency of events, the synchrony between the individuals during the event, the closest approach between the individuals, and the occurrence of joint travel. When considering higher-order patterns of fission–fusion dynamics, our results suggest that independent movement decisions, especially to and from the den, play a large role in influencing social encounters, but that factors beyond independent movement decisions – most likely social processes – underlie variation in what happens after hyenas encounter one another.
Our findings—combined with other work in this species on long-distance recruitment (Gersick et al. 2015), cooperative mobbing of lions (Montgomery et al. 2023), and communal territory defense (Boydston et al. 2001)—suggest that hyenas pursue a mixed strategy for acquiring critical social-interaction time with important partners, leaning on the passive mechanism of chance encounters with groupmates both at and away from dens, but also actively pursuing convergence with particular individuals, especially during the more dispersed, travel-heavy phases of the daily routine. A productive avenue for future work will be to dive deeper into functional differences between post-merging behavior resulting from merges that occur at dens compared to away from dens. For instance, prior work has suggested that groups of hyenas congregating at dens or other resting places often subsequently engage in cooperative behaviors such as group hunting or territorial border patrolling (Kruuk 1972; Mills 1990; Holekamp et al. 1997a). The unprecedented spatiotemporal detail from data collected using biologgers as implemented here therefore offers potential for new perspectives on the catalysis and dynamics of these cooperative behaviors.
Future work notwithstanding, results from this study have important implications for hyena social structure, particularly in cases where a single group of hyenas has multiple active communal dens, as sometimes occurs (Fig. S2). Our results suggest that the use of multiple communal dens should drive more modular social structure. In fact, each of the four permanent group fission events documented in our study population since 1988 (e.g., Holekamp et al. 1993) was preceded by use of multiple communal dens (personal observation), suggesting that this enhanced modularity may have important consequences for the fates of entire hyena societies.
The small number of tagged individuals limits some of the interpretation of the results of the current study. With only a small subset of one social group monitored, it cannot be confidently concluded that the patterns observed here are characteristic of spotted hyena fission–fusion patterns more broadly. Another limitation arising from our small sample size is that we lack information on the behavior of other members of the group during merge-split events, so we are unable to account for the effects or subgroup structure or composition on the behavior of our tagged individuals. Additionally, we tagged individuals of diverse dominance ranks, and because rank is closely associated with kinship and kin tend to form the strongest social bonds, our study individuals were not closely bonded (see Holekamp et al. 1997a; Smith et al. 2007, 2008, 2010) for relationships between rank and hyena association patterns). It remains unclear to what extent our results will generalize to more closely bonded individuals. Finally, the small number of tagged individuals precluded us from investigating questions about how individual attributes or long-term social relationships influenced fission–fusion dynamics.
Despite these limitations resulting from a small sample size of individuals, this work is nevertheless instructive about the nature of hyena fission–fusion dynamics. Although we only tagged a few individuals, each of these hyenas was monitored continuously and simultaneously, providing an unprecedented dataset on the dynamics of association that included an order of magnitude more observations of social encounters than traditional observations of the same individuals over the same period, and included observations at times in which observations are not typically carried out (e.g., middle of the night). The analyses conducted here harness the strengths of this dataset to quantify the properties of social encounters, and these analyses complement prior work on this species in a larger set of individuals. For instance, hyena communal dens have often been described as social hubs (Holekamp et al. 1997a), but prior work has not been able to quantify how communal dens influence fission–fusion dynamics. Our work supports the role of dens as important social hubs and clarifies how this operates—dens are more typically meet-up points facilitating joint travel then destinations of jointly traveling individuals. Future work should aim to deploy tags on many more individuals concurrently, ideally on every member of a social group. Doing so would also allow for the expansion of the approach presented here from dyadic merge-split events to polyadic events and the fission–fusion dynamics of the group as a whole. Finally, hyenas are known to use long-distance vocalizations to recruit their clan-mates over large distances in contexts requiring collective action (Gersick et al. 2015), so the role of communication in driving fission–fusion patterns also warrants further investigation.
This study also provides methodological insight into how to study social behavior in systems with fission–fusion dynamics. We suggest that a useful approach is to distinguish drivers of social encounters from drivers of social interaction – that is, an explicit distinction between the processes that drive (1) when, where, and which conspecifics individuals encounter (2) post-fusion behavior and (3) how and why they part ways. Our three-phase model provides a useful tool for asking these questions by offering a means to identify and measure the merging, together, and splitting up components of encounters between individuals. Furthermore, the taxonomy of event types derived from these phases facilitates understanding of processes operating across phases. For instance, our analysis of the frequency of different event types revealed a fundamental asymmetry between splits and merges (Fig. 3B), indicating that splits are not simply merges in reverse. This asymmetry likely derives from key differences in the ways in which animals meet up vs split apart, including the communication mechanisms involved in these changes. Although we focused here on dyadic interactions, the method of characterizing merge-split events could also be extended beyond a dyadic level to characterize higher-order fission–fusion dynamics of subgroups. Our approach provides a generalizable framework for future work investigating fission–fusion dynamics across different contexts, social groups, species, and spatial scales.
Finally, the dual role of communal dens as physical and social resources suggests a potentially broadly-acting process by which spatial and social heterogeneity become aligned. By attracting individuals or promoting social interactions, spatiotemporally predictable resource hotspots become social hotspots, leading individuals to use these locations for social purposes. Through this “social piggybacking” effect, the social landscape conforms to the physical landscape, and socially-driven and resource-driven movements produce the same behavior. The communal den in spotted hyenas is a clear example of this process: the physical resource is only useful to a subset of individuals (mothers with den-dependent offspring), yet non-reproductive individuals frequently visit, demonstrating that this physical resource has become a social resource. Other examples include foraging glades in vulturine guineafowl that serve as a hotspot of social interactions among groups (Papageorgiou et al. 2019), and social hotspot trees that are sites of predictable large gatherings of wild zebra finches (Loning et al. 2023). Future work should explore the role of social hotspots in shaping fission–fusion dynamics in other species. Foraging sites, watering holes, resting sites, sunny/shady locations, or locations with good visibility for vigilance are all examples of spatiotemporally predictable physical resources that can become social resources. This concept of social piggybacking highlights how animal movement patterns need to be understood at the spatial-social interface, a framework that views spatial and social phenotypes as simultaneously arising and causally intertwined (Webber et al. 2023).
Data availability
Raw movement and accelerometer data used in this study are publicly available from Dryad data repository (Strauss et al. 2021). All analysis code required to replicate the results is available at https://github.com/arianasp/hyena_fission_fusion.
References
Archie EA, Moss CJ, Alberts SC (2006) The ties that bind: Genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc R Soc B 273:513–522. https://doi.org/10.1098/rspb.2005.3361
Aureli F, Schaffner CM, Boesch C et al (2008) Fission-fusion dynamics: new research frameworks. Curr Anthropol 49:627–654
Boydston EE, Morelli TL, Holekamp KE (2001) Sex differences in territorial behavior exhibite4d by the spotted hyena (Hyaenidae, Crocuta crocuta). Ethology 107:369–385. https://doi.org/10.1046/j.1439-0310.2001.00672.x
Brown AK, Pioon MO, Holekamp KE, Strauss ED (2021) Infanticide by females is a leading source of juvenile mortality in a large social carnivore. Am Nat 198:642–652. https://doi.org/10.1086/716636
Chapman CA, Chapman LJ, Wrangham RW (1995) Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behav Ecol Sociobiol 36:59–70. https://doi.org/10.1007/BF00175729
Conradt L, Roper TJ (2005) Consensus decision making in animals. Trends Ecol Evol 20:449–456. https://doi.org/10.1016/j.tree.2005.05.008
Farine DR, Whitehead H (2015) Constructing, conducting and interpreting animal social network analysis. J Anim Ecol 84:1144–1163. https://doi.org/10.1111/1365-2656.12418
Farine DR, Firth JA, Aplin LM et al (2015) The role of social and ecological processes in structuring animal populations: A case study from automated tracking of wild birds. R Soc Open Sci 2:150057. https://doi.org/10.1098/rsos.150057
Firth JA, Sheldon BC (2015) Experimental manipulation of avian social structure reveals segregation is carried over across contexts. Proc R Soc B 282:20142350. https://doi.org/10.1098/rspb.2014.2350
Gersick AS, Cheney DL, Schneider JM, Seyfarth RM, Holekamp KE (2015) Long-distance communication facilitates cooperation among wild spotted hyaenas, Crocuta crocuta. Anim Behav 103:107–116. https://doi.org/10.1016/j.anbehav.2015.02.003
Green DS, Johnson-Ulrich L, Couraud HE, Holekamp KE (2018) Anthropogenic disturbance induces opposing population trends in spotted hyenas and African lions. Biodivers Conserv 27:871–889. https://doi.org/10.1007/s10531-017-1469-7
He P, Klarevas-Irby JA, Papageorgiou D, Christensen C, Strauss ED, Farine DR (2022) A guide to sampling design for GPS-based studies of animal societies. Methods Ecol Evol 14:1887–1905. https://doi.org/10.1111/2041-210X.13999
Hobson EA, Silk MJ, Fefferman NH, Larremore DB, Rombach P, Shai S, Pinter-Wollman N (2021) A guide to choosing and implementing reference models for social network analysis. Biol Rev 96:2716–2734. https://doi.org/10.1111/brv.12775
Holekamp KE, Smale L (1998) Behavioral development in the spotted hyena. Bioscience 48:997–1005
Holekamp KE, Strauss ED (2020) Reproduction within a hierarchical society from a female’s perspective. Integr Comp Biol 60:753–764. https://doi.org/10.1093/icb/icaa068
Holekamp KE, Ogutu JO, Dublin HT, Frank LG, Smale L (1993) Fission of a spotted hyena clan: consequences of prolonged female absenteeism and causes of female emigration. Ethology 93:285–299. https://doi.org/10.1111/j.1439-0310.1993.tb01210.x
Holekamp KE, Cooper SM, Katona CI, Berry NA, Frank LG, Smale L (1997a) Patterns of association among female spotted hyenas (Crocuta crocuta). J Mammal 78:55–64. https://doi.org/10.2307/1382638
Holekamp KE, Smale L, Berg R, Cooper SM (1997b) Hunting rates and hunting success in the spotted hyena (Crocuta crocuta). J Zool 242:1–15. https://doi.org/10.1111/j.1469-7998.1997.tb02925.x
Holekamp KE, Smith JE, Strelioff CC, Van Horn RC, Watts HE (2012) Society, demography and genetic structure in the spotted hyena. Mol Ecol 21:613–632. https://doi.org/10.1111/j.1365-294X.2011.05240.x
Johnson MP, Tyack PL (2003) A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J Ocean Eng 28:3–12. https://doi.org/10.1109/JOE.2002.808212
Johnson M, De Soto NA, Madsen PT (2009) Studying the behaviour and sensory ecology of marine mammals using acoustic recording tags: A review. Mar Ecol Prog Ser 395:55–73. https://doi.org/10.3354/meps08255
Kelley JL, Morrell LJ, Inskip C, Krause J, Croft DP (2011) Predation risk shapes social networks in fission-fusion populations. PLoS ONE 6:e24280. https://doi.org/10.1371/journal.pone.0024280
Kruuk H (1972) The Spotted Hyena: A Study of Predation and Social Behavior. University of Chicago Press, Chicago, IL
Lehmann KDS, Montgomery TM, MacLachlan SM, Parker JM, Spagnuolo OS, VandeWetering KJ, Bills PS, Holekamp KE (2017) Lions, hyenas and mobs (Oh my!). Curr Zool 63:313–322. https://doi.org/10.1093/cz/zow073
Loning H, Fragueira R, Naguib M, Griffith SC (2023) Hanging out in the outback: the use of social hotspots by wild zebra finches. J Avian Biol 2023:e03140. https://doi.org/10.1111/jav.03140
Lukas D, Clutton-Brock T (2018) Social complexity and kinship in animal societies. Ecol Lett 21:1129–1134. https://doi.org/10.1111/ele.13079
Mann J, Connor RC, Tyack PL, Whitehead H (eds) (2000) Cetacean societies: field studies of dolphins and whales. University of Chicago Press, Chicago, IL
Marlowe FW (2005) Hunter-gatherers and human evolution. Evol Anthropol 14:54–67. https://doi.org/10.1002/evan.20046
Mills MGL (1990) Kalahari Hyenas: Comparative Behavioral Ecology of Two Species. The Blackburn Press, Caldwell, NJ
Montgomery TM, Lehmann KDS, Gregg S, Keyser K, McTigue LE, Beehner JC, Holekamp KE (2023) Determinants of hyena participation in risky collective action. Proc R Soc B 290:20231390. https://doi.org/10.1098/rspb.2023.1390
Mourier J, Vercelloni J, Planes S (2012) Evidence of social communities in a spatially structured network of a free-ranging shark species. Anim Behav 83:389–401. https://doi.org/10.1016/j.anbehav.2011.11.008
O’Mara M, Dechmann DKN (2023) Greater spear nosed bats commute long distances alone, rest together, but forage apart. bioRxiv, https://doi.org/10.1101/2021.09.30.462631
Papageorgiou D, Christensen C, Gall GEC, Klarevas-Irby JA, Nyaguthii B, Couzin ID, Farine DR (2019) The multilevel society of a small-brained bird. Curr Biol 29:R1120–R1121. https://doi.org/10.1016/j.cub.2019.09.072
Ramos-Fernandez G, King AJ, Beehner JC et al (2018) Quantifying uncertainty due to fission–fusion dynamics as a component of social complexity. Proc R Soc B 285:20180532. https://doi.org/10.1098/rspb.2018.0532
Ripperger SP, Carter GG (2021) Social foraging in vampire bats is predicted by long-term cooperative relationships. PLoS Biol 19:e3001366. https://doi.org/10.1371/journal.pbio.3001366
Schamberg I, Cheney DL, Clay Z, Hohmann G, Seyfarth RM (2017) Bonobos use call combinations to facilitate inter-party travel recruitment. Behav Ecol Sociobiol 71:75. https://doi.org/10.1007/s00265-017-2301-9
Sikes RS, Gannon WL (2011) Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 92:235–253. https://doi.org/10.1644/10-MAMM-F-355.1
Silk MJ, Croft DP, Tregenza T, Bearhop S (2014) The importance of fission-fusion social group dynamics in birds. Ibis 156:701–715. https://doi.org/10.1111/ibi.12191
Smith JE, Memenis SK, Holekamp KE (2007) Rank-related partner choice in the fission–fusion society of the spotted hyena (Crocuta crocuta). Behav Ecol Sociobiol 61:753–765. https://doi.org/10.1007/s00265-006-0305-y
Smith JE, Kolowski JM, Graham KE, Dawes SE, Holekamp KE (2008) Social and ecological determinants of fission–fusion dynamics in the spotted hyaena. Anim Behav 76:619–636. https://doi.org/10.1016/j.anbehav.2008.05.001
Smith JE, Van Horn RC, Powning KS, Cole AR, Katharine GE, Memenis SK, Holekamp KE (2010) Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav Ecol 21:284–303. https://doi.org/10.1093/beheco/arp181
Spiegel O, Leu ST, Sih A, Bull CM (2016) Socially interacting or indifferent neighbours? Randomization of movement paths to tease apart social preference and spatial constraints. Methods Ecol Evol 7:971–979. https://doi.org/10.1111/2041-210X.12553
Strauss ED, Holekamp KE (2019) Social alliances improve rank and fitness in convention-based societies. P Natl Acad Sci USA 116:8919–8924. https://doi.org/10.1073/pnas.1810384116
Strauss ED, Jensen FH, Gersick AS, Thomas M, Holekamp KE, Strandburg-Peshkin A (2021) Daily ranging and den usage patterns structure fission-fusion dynamics and social associations in spotted hyenas. Dryad. https://doi.org/10.5061/dryad.0p2ngf22k
Van Horn RC, Engh AL, Scribner KT, Funk SM, Holekamp KE (2004) Behavioural structuring of relatedness in the spotted hyena (Crocuta crocuta) suggests direct fitness benefits of clan-level cooperation. Mol Ecol 13:449–458. https://doi.org/10.1046/j.1365-294X.2003.02071.x
Webber QMR, Albery GF, Farine DR, Pinter-Wollman N, Sharma N, Spiegel O, Vander Wal E, Manlove K (2023) Behavioural ecology at the spatial–social interface. Biol Rev 98:868–886. https://doi.org/10.1111/brv.12934
Wilkinson GS, Carter G, Bohn KM et al (2019) Kinship, association, and social complexity in bats. Behav Ecol Sociobiol 73:7. https://doi.org/10.1007/s00265-018-2608-1
Acknowledgements
We thank the Kenya Wildlife Service, the Kenyan Wildlife Training and Research Institute, the Narok County Government, the Kenyan National Committee on Science, Technology and Innovation, the Naboisho Conservancy, the Mara Conservancy, and Brian Heath for permissions to conduct this research. Thanks to many current and former members of the Mara Hyena Project for detailed data collection. We thank Benson Pion, Rebecca LaFleur, and Morgan Lucot for assistance in the field during collar deployment. We thank Mark P. Johnson for providing the DTAG tag technology and contributing to collar development and testing as well as discussions about ranging patterns and den use. We also thank the editor, two anonymous reviewers, Alison Ashbury, Pranav Minasandra, and the members of the Communication and Coordination Across Scales team for feedback and useful discussion.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by Human Frontier Science Program award RGP0051/2019 to AS-P and KEH, National Science Foundation grants OISE1853934 and IOS1755089 to KEH, and Alexander von Humboldt Postdoctoral Fellowship to EDS. This work was also supported in part by National Science Foundation Grant OIA 0939454 (Science and Technology Centers) via “BEACON: An NSF Center for the Study of Evolution in Action” and by a grant to FHJ from the Carlsberg Foundation. AS-P received additional funding from the Gips-Schüle Stiftung, the Zukunftskolleg at the University of Konstanz, and the Max Planck Institute of Animal Behavior, and was in part funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC 2117 – 422037984.
Author information
Authors and Affiliations
Contributions
ASG, FHJ, AS-P, and KEH conceived the hyena collaring study, secured funding, and collected the tracking data that forms the basis for this work. KEH maintains the long-term study of spotted hyenas that enabled this work, and worked with FHJ to integrate and test the tracking collars. EDS and AS-P conceived the specific questions addressed by this work, designed the analyses, wrote the analysis code, and produced a first draft of the manuscript. MT conducted a thorough code review to ensure the accuracy and reproducibility of analyses and implemented improvements to the analysis. All authors contributed to, and approved, the final manuscript.
Corresponding author
Ethics declarations
Significance Statement
Spatial proximity with others forms the foundation of animal social environments. Consequently, in species where spatial proximity among individuals or groups is fluid, the processes by which animals split up and come together are critical determinants of how animals experience their worlds. We use simultaneous GPS tracking to examine the dynamics of merging and splitting events among pairs of spotted hyenas, and present a general framework for classifying these events based on dyadic movement patterns. We find that many quantitative measures of these events are explained by travel to and from a key location in the landscape: the group’s communal den. Our results demonstrate how social association is structured by the location of physical resources, which can become social hotspots that draw individuals to use these locations for social purposes.
Compliance with ethical standards
All field methods were approved by Kenya Wildlife Service under permit KWS/BRM/5001 to KEH. They conform to guidelines published by the American Society of Mammalogists (Sikes and Gannon 2011), and they were also approved by the IACUC at Michigan State University under approval PROTO201900126, which was issued most recently in January, 2020.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by K. Eva Ruckstuhl.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strauss, E.D., Jensen, F.H., Gersick, A.S. et al. Daily ranging and den usage patterns structure the spatiotemporal properties of social encounters in spotted hyenas. Behav Ecol Sociobiol 78, 45 (2024). https://doi.org/10.1007/s00265-024-03458-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03458-3