Abstract
Most animal behaviors show large within- and among-individual variation, and this includes competitive male behaviors. With male fighting for example, aggressiveness often correlates with dominance, and contest duration varies with age. However, few studies have directly quantified how mean aggressiveness and contest duration, the variation among individuals in both traits, and the relationship among them, vary with age. Here we address these gaps and examine the effect of male age and genotype on two key aspects of male fighting behavior - aggressiveness (here measured as latency to fight) and contest duration - and the relationship between them. We do this using isogenic lines of the broad-horned flour beetle Gnatocerus cornutus. We observed fighting behavior of paired males of similar body size and age. Using uni- and multivariate mixed models, we show that although there was a significant difference between younger and older males in contest duration, mean aggressiveness was not affected by male age. However, the variation in aggression and fight duration varied with age, being greater in younger and older males respectively. Additionally, although there was a positive correlation between aggressiveness and contest duration in younger males, this relationship was not found in older males. Finally, the only significant genetic effect was for aggression in younger males. Our study shows that age differentially shapes key components of male fighting behavior as well as the relationship among them, highlighting the dynamic nature and context-dependence of fighting.
Significance statement
We examined the effect of male age and genotype on two key aspects of male fighting behavior - aggressiveness (here measured as latency to fight) and contest duration - and the relationship between them. Importantly, we provide statistical methods that enable untangling these effects that will be useful to others. We used isogenic lines of the broad-horned flour beetle Gnatocerus cornutus, and observed fighting behavior of paired males of similar body size and age. We show that age differentially shapes key components of male fighting behavior as well as the relationship among them, and only find genetic effects for aggression in younger males. These novel results highlight the context dependent nature of fighting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One behavior frequently correlated with male fitness is fighting (Darwin 1871; Andersson 1994; Emlen 2008; Thornhill and Alcock 2013). In many animals, males fight for access to resources, including females (mating opportunities), and winning contests increases male reproductive success. More aggressive males engage in contests faster or more often (Benus et al. 1991; de Boer et al. 2003), but the duration of each fight will depend on an individual’s cost threshold – the point at which costs can no longer be met (Taylor and Elwood 2003). Males with lower cost thresholds will fight for less time and are defeated more quickly than males with higher thresholds, who are more persistent in contests (Taylor et al. 2001; Prenter et al. 2006). Aggression and fight duration can also depend on the value of the resource being fought over, with extended and escalated contests more likely with more valuable resources (Batchelor and Briffa 2010). However, because greater aggressiveness and longer contest duration increase the risk of injury, and are costly in time and energetic terms (Glass and Huntingford 1988; Kelly and Godin 2001; Briffa and Elwood 2005), the balance between the benefits of winning and cost of continued fighting should determine optimal male fighting behavior (Smith and Parker 1976; Parker and Smith 1990). In addition to these intrinsic (e.g. cost threshold) and extrinsic (e.g. resource value) individual-level sources of variation in fighting behavior, elements of fighting represent a shared-phenotype, with both fighting behavior and fight outcomes for the focal individual being dependent on the opponent and its behavior (Lane et al. 2020).
Like other behaviors, the costs and benefits of male fighting are expected to vary with age. Hence the shape of the trade-offs between reproduction and survival, and between current and future investment, will vary with age (Rose 1991; Arking 1998; Hillesheim and Stearns 1992; Grotewiel et al. 2005)- and this variation may be adaptive. For example, fighting may be more costly in younger males because of their greater future reproductive potential (a phenomenon also called the “asset protection” principle). Hence, older males may have a higher individual cost threshold than younger males, and as a result, older males could fight for longer or start fights sooner (Kemp 2006), placing them at an advantage when facing younger counterparts.
Furthermore, resource holding potential (RHP) (the ability to win fights: Parker 1974) or the value of the resource to the individual may increase with age (Harley et al. 1994). In northern elephant seals (Mirounga angustirostris), for example, older males have higher win rates in male-male fights than younger males (at least for a period in their mature adulthood), indicating that they have acquired the necessary means for winning contests (i.e., large body size). While this could be part of an overarching life history strategy, it ultimately is age-related variation in RHP that decides contest outcome (Kemp 2000, 2002). Similarly, older male broad-horned flour beetle Gnatocerus cornutus win more fights, which suggests resource value or fighting ability increases with age (Okada et al. 2020). Alternatively, older males may have lower resource holding potential as performance declines with age, and hence younger males could be more aggressive (Kemp 2006). It should however be noted that the estimated effect of age will depend on definition of ‘young’ and ‘old’.
Behaviors are often correlated and repeatable within individuals (Sih et al. 2004; Réale et al. 2007). For example, in the wild boar (Sus scrofa), more aggressive males more readily engage in a contest and fight more frequently (Bolhuis et al. 2005; Camerlink et al. 2015) and for longer (Camerlink et al. 2015) than less aggressive males. If behaviors vary with age, then the correlations among these behaviors could also vary with age. However, few studies have investigated how correlations among different fighting behaviors, such as the relationship between aggressiveness and contest duration, vary with age.
Here we quantified the effect of age on aggressiveness, defined as the latency to start a fight, and fight duration in G. cornutus. These beetles use their enlarged mandibles to engage in fights over access to females and males that win fights secure matings (Okada et al. 2006). When placed in a small container, males use their well-developed mandibles against other males, even in the absence of females (Okada et al. 2006). Typically the larger male wins the fight, and the loosing males flees the fighting area and becomes non-aggressive for four days (Okada and Miyatake 2010). Previous work has found that while older males are more likely to win fights, they do not start fights earlier or fight for longer (Okada et al. 2020). However, whether the relationship between latency and duration of male-male fighting varies with age has not been investigated previously, and little is known about the genetic basis of fighting behavior or fight outcome.
Isofemale lines, effectively representing distinct genotypes, are commonly used to investigate the genetic basis of traits (Hoffmann and Parsons 1989; David et al. 2005), especially when avoiding breaking naturally occurring linkage patterns (Hosken and Wilson 2019), and for replicating genotypes to allow for repeated testing without confounding prior experience with the question of interest (e.g., Ingleby et al. 2013). Here we used isolines to test for the role of genetic variation in shaping variation in fight latency and duration, and test for genetic associations between these components of fighting behavior (cf. Matsumura et al. 2020). Additionally, while Okada et al.’s (2020) ‘old’ males were 105 days old, here our ‘old’ males were 50 days old. At this age (50 days) selective disappearance – the loss through death of certain phenotypes which can be problematic in studies of age effects (Bouwhuis and Vedder 2017) is not likely as the maximum lifespan of the beetles is about 140 days in the lab and beetle death rates are low before 50 days old. Hence it is unlikely that the ‘old’ sample in our study contains a subset of only highly performing genotypes (for further discussion of selective disappearance, see Bouwhuis and Vedder 2017). We measured time to contest commencement (fight latency) as aggressiveness (Camerlink et al. 2015) - more aggressive individuals start a fight sooner - and contest duration as a measure of an individual’s cost threshold. We reasoned that older males should invest more in male-male combat than younger males, and hence predicted that older males would show greater aggressiveness and longer contest duration (Kemp 2006). Furthermore, we expected that the relationship among fighting behaviors would vary with age, like many other behaviors (e.g., MacNulty et al. 2009), with young males having a longer latency to fight and shorter fight duration.
Materials and methods
Insects and culture
The stock population of G. cornutus used in this study has been maintained in the laboratory for over 50 years. Animals were reared in incubators under 16 L:8D light conditions (7:00 light on, 23:00 light off) at 25℃. We used a mixture of whole grain flour and beer yeast (19:1) as food. Last instar larvae were collected from laying pots and placed individually into 24-well plates to allow pupation. When the pupae hatched, we fed the adult beetles and reared them as described above until the start of the experiment. In this study, we used 18 iso-female lines established from the stock population (Matsumura et al. 2020). Initially, 18 males and 18 females were selected at random and paired. Subsequently, full-sib matings within each family were used to propagate each line for over 40 generations until the present study was conducted. Isolines represent distinct genotypes, and the variation among isolines can therefore provide insight into the genetic variation underlying various characters among individuals in the base population that these lines were derived from (Hoffmann and Parsons 1989). Previous work has shown these isolines capture significant genetic variation (e.g. Matsumura et al. 2020).
As a measure of body size, we used mid-prothorax width (± 0.01 mm) of males using the dissecting microscope monitoring system (see Fig. 1, in Okada and Miyatake 2009). To remove the confounding effect of body size (Okada et al. 2006), opponents’ body sizes were matched. In this study, we used 10-day old (‘young’) and 50-day old (‘old’) males. We chose 50 days to minimize problems associated with selective disappearance that can significantly impact studies of aging effects (e.g., Curio 1983; Bouwhuis and Vedder 2017). Our beetles live on average about 110 days, and 50 days represents about half their average lifespan (see Methods). Most individuals (> 97%) survive to this age (S. Nishitani personal observation; and see Okada et al. 2020). Thus, although strictly 50 days should be referred to as middle-aged, here we will refer to them as ‘old’ to distinguish them from the 10-day old ‘young’ males.
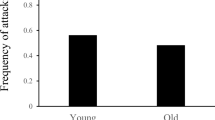
Effects of male age on the latency (A) and duration (B) of fighting in G. cornutus. While male age did not affect how long it took for fights to begin, it did affect the duration of fights, with older males fighting for longer. Furthermore, the variance in latency was smaller in older males, whereas the variance in duration was larger among older males
Fighting behavior
Only males with no fighting experience were used in the experiment. An individual’s fighting ability depends on the phenotype of its opponent (Lane et al. 2020), and experimentally there is no perfect solution to this dependency – neither random opponent allocation nor the use of standardized opponents are perfect solutions to this social-environment effect, especially when fighting outcomes are non-transitive. Here we chose to fight males against opponents from the same age and isoline as themselves. That is, each male fought against a genetically identical opponent of the same age. To control for the effect of body size on fighting success, males were paired so that the difference in body size between contestants was less than 0.01 mm (Okada and Miyatake 2010). Thus, males were both phenotypically and genetically matched.
Two males were simultaneously placed on a sheet of filter paper in a well (17 mm diameter, 20 mm high) of 48-well plate (Cell Star, Greiner Bio-One, Kremsmünster, Austria). Trials were then continuously monitored until fight outcomes could be scored. In the present study, we defined the winner and loser following methods described in Okada and Miyatake (2009); the winner was the male that pushed his opponent out of the fight site and chased him. The loser was the male that retreated from the fight site (Okada et al. 2006). We analysed the latency of the contest (i.e., how long it took before the two males initiated a fight: aggression) and the contest durations (i.e., time from first attack to the end of fighting). Trials lasted up to 30 min. We observed 3–10 pairs from each isoline (total; 10 days old: n = 87 pairs, 50 days old: n = 128; see supplementary Table 1). All observations were conducted in a room maintained at 25 °C between 12:00 to 19:00.
Statistical analyses
We tested for differences in mean latency and duration between young and old males in separate univariate generalized linear mixed models. Latency and duration measure the time elapsed until the start and the end of a fight, respectively, and are positive non-integer values that show a right-skewed distribution (Fig. 1). We therefore assumed a Gamma distribution and used a log link function using the glmer function in the R package lme4 1.1–31 (Bates et al. 2015) in R 4.2.2 (R Core Team 2017). Separate models with age as a categorical fixed effect and isoline as a random effect were fitted to both latency and duration. The statistical significance of the fixed effect of age class on both traits was based on a likelihood-ratio test.
Second, we fitted four separate trait- and age-specific models to obtain trait and age-specific estimates of the variance among versus within isolines, again using generalised linear mixed models with isoline as a random effect. The proportion of variance explained by isoline in each of the four ‘traits’ (latency and duration at young and old age) was calculated following Nakagawa et al. (2017): The variance explained by isoline was divided by the sum of the variance explained by isoline and the residual (or observation-level) variance, and the latter was obtained using the trigamma function. P-values for the variances explained by isoline were based on likelihood ratio tests and halved following Self and Liang (1987).
Third, we tested if the variance components estimated by these univariate models differed significantly between age classes using two bivariate mixed models in ASReml-R v 4.1.0.176 (Butler 2022), with either the latency for young and old males, or the duration for young and old males, as the two response variables. We used likelihood-ratio tests to compare models that estimate age-specific variances to models in which these have been constrained to be identical. At this stage, we did not model any correlations among traits. Because at the time of writing ASReml-R cannot fit multivariate models with more than one non-Gaussian trait (Salvador Gezan, VSN Support, pers. comm.), we used log-transformed traits and Gaussian error families for these models. Although we can therefore not directly compare the multivariate variance components to those estimated above, they were found to be qualitatively similar to those provided by the univariate Gamma models.
Fourth, we estimated the correlations between latency and duration in both age classes, again within and across isolines, within a single multivariate model. To test if each correlation was significantly different from zero, we compared this unconstrained model to a model in which the correlation was constrained to zero. This was complemented by a test if these correlations differed from each other (i.e., among age classes), by comparing the full model to a model in which correlations were constrained to be identical. Because for each male we have a measurement of latency and duration only for a single age class, the within-line correlations between age classes (e.g., latency young versus old) are not estimable and were therefore always constrained to zero.
Ethical note
For the experiments on fighting behavior, the insects were reared and tested in a manner that did not stress them according to Guidelines for the ethical treatment of nonhuman animals in behavioral research and teaching. The experimental animals used were bred in captivity. The rearing cages were managed in the same manner as in previous studies, and we did not induce any pain.
Results
The mean latencies to engage in a fight (i.e., aggressiveness) for young and old males were 399 s and 395 s, respectively, and were not significantly different from each other (b ± s.e. = 0.038 ± 0.108, χ21 = 0.13, P = 0.72; Fig. 1A). However, on average fights between young males were significantly shorter than fights between old males (15.8 and 36.7 s, respectively; b ± s.e. = 0.81 ± 0.13, χ21 = 35.6, P < 0.001; Fig. 1B). Note that model estimates are on the log-link scale.
As illustrated in Fig. 1, there is a large amount of variation in both latency and duration within both age classes, and the variance in (log-transformed) fight latency and duration differs significantly between both age classes. Whereas the variance in log-transformed latency measured in seconds is significantly higher among young males (1.00) than among old males (0.46; χ21 = 15.8, P < 0.001), the variance in fight duration is significantly lower among young males (0.68) compared to old males (1.05; χ21 = 4.6, P = 0.032).
Of the variance in latency among young males, a statistically significant 18% was attributable to isoline (χ20/1 = 8.5, P = 0.002). In contrast, the variance explained by isoline was much smaller and non-significant for the latency of old males (3%; χ20/1 = 0, P = 1), and the duration of fights between either young (5%, χ20/1 = 0, P = 1) or old males (7%, χ20/1 = 2.7, P = 0.051).
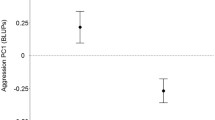
As there is only evidence for variation among isolines for fight-latency in young males, and maybe duration in old males, among-line correlations (genetic correlations) between (log-transformed) age-specific latency and duration are not estimable. Looking within lines (testing for phenotypic correlations), there was a statistically significant positive correlation between log-transformed latency and duration in young males (Fig. 2A: r ± s.e. = 0.32 ± 0.10, χ21 = 8.9, P = 0.003). The correlation was much weaker and non-significant in older males (Fig. 2B: r ± s.e. = 0.025 ± 0.089, χ21 = 0.08, P = 0.78), and significantly weaker than the correlation in young males (Fig. 2B: χ21 = 4.7, P = 0.031).
Discussion
There have been few investigations into the impact of age on fighting behaviors and the within-individual associations between fighting behaviors. We tested for such effects here using horned flour-beetle isolines. Our main findings were that age affected contest duration, with older males fighting for longer, but there were no effects of age on aggression - the time it took for individuals to engage in a fight (fight-latency). We also found limited effects of genotype, with fight latency at young age being the only trait with substantial genetic variation, and it was only at young age that we saw phenotypic correlations between fight latency and duration. We discuss these findings in turn.
Older males fought for longer than younger males and there was more variation in the duration of their contests. This is broadly consistent with theoretical predictions that a “late-hawk” strategy (act more aggressively at old age) is common across broad contest-model parameter space (Kemp 2006). A possible reason for the mean duration increasing with age is that the threshold at which an individual stops competing – the individual cost threshold –of older males is higher as they will have fewer opportunities to engage in future reproductive competitions (and beetles fight for access to females). That is, the opportunity costs of fighting are lower for older males, and they hence value winning current fights more than younger males. Life history theory predicts increased investment in current activities as future investment opportunities decline (Stearns 1992), and this prediction has empirical support (Magnhagen and Vestergaard 1991; Candolin 1998; Poizat et al. 1999; Kemp 2002). Our results are broadly consistent with this fundamental conjecture, and with findings for female wasps (Goniozus nephantidis). In this species, older females win fights over prey-items, presumably because they value winning more (Humphries et al. 2006).
Not all studies find age effects on contest duration (e.g., Lee et al. 2014). For example, previous work with G. cornutus did not find a difference between young and old male fight durations, although it did find that older males win more fights (Okada et al. 2020). Okada et al. (2020) however used males that were significantly older than the old males we used here, and opponents were selected at random and not from the same isoline as in the present study (also see Brandt 1999). If there was selective disappearance during aging (Bouwhuis and Vedder 2017), the former could account for differences between the two studies, with for example only superior genotypes living to very advanced age and winning more fights without having to fight for longer (Okada et al. 2020). Alternatively, our male-matching protocol (males fought with related same-age and -sized males (i.e. from the same isoline) may have impeded detection of age effects because males probably had very similar resource holding potential. Investigating the importance of asymmetric resource holding potential is something for future consideration. Close ability matches often leads to longer fights (Austad 1983, Jonart et al. 2007), and there is a positive relationship between relatedness and fight duration in anemones (Lane et al. 2020). This, coupled with age differences in future opportunities/value of winning (Stearns 1992), probably explains why we revealed a difference not previously detected (Okada et al. 2020).
It is not entirely clear why there was greater variation in fight duration in older males. If older males had experienced (more) previous fights, this would provide one explanation for the difference, but the beetles used here had no previous exposure to fighting. It may be that within-individual variation generally increases over time because of stochastic environmental or mutational effects and that explains the increased variance in older males. Be that as it may, this would not explain the opposite effect noted for aggressiveness (latency to fight), which did not vary with age, but the variance in aggression was greater in younger males. This average effect is consistent with previous work on G. cornutus that found that the likelihood of fight initiation did not vary with age (Okada et al. 2020; also see Okada et al. 2019), but deviates from our a priori expectations – we expected aggression to also increase with age for the reasons outlined above with respect to fight duration (and see e.g., predictions from Kemp 2006). One possibility is that aggressiveness is independent of resource holding potential and/or resource value. In wild boar, aggressiveness does not affect the outcome of a contest (Camerlink et al. 2015), which is consistent with the absence of an association between the value of aggression and/or fighting ability. However, other studies have shown that measures of aggression are correlated with estimated resource holding potential and other factors, like stage of the breeding cycle (e.g., Jonart et al. 2007). In any case, we see no effect of age on aggression, mirroring work with burying beetles Nicrophorus vespilloides (Lee et al. 2013). Whether this is a general pattern and precisely why there was no effect remains to be established. Additionally, the greater variation in aggression in younger males may reflect variation in how well they had been able to accrue and assimilate resources, with older males all having had sufficient time to accumulate what they need, resulting in lower variation in aggression. It is worth noting that our methods mean selective disappearance (Bouwhuis and Vedder 2017) is unlikely to explain the reduction in variation we see at older age. In the present study, no individuals were assayed as both young and old males, and so age-associated changes in contest behavior were not explicitly measured. This would be an interesting area for future study.
A significant positive non-genetic association (i.e., within lines) between aggression and duration of fighting was found in younger, but not older males. Thus, at young age, fighting behavior is phenotypically integrated to an extent - and could be thought of as a young-male behavioral syndrome (Briffa et al. 2015) - but the association breaks down at older age. This pattern mirrors that seen in hermit crabs Pagurus bernhardus where aggression correlates with other behaviors in some (environmental) conditions, but not others (Mowles et al. 2012). Similarly, in fallow deer (Dama dama) individuals vary in the consistency of their fighting behaviors (Jennings et al. 2013). The lack of an association between aggressiveness and contest duration in older males also reflects findings in wild boar (Camerlink et al. 2015). There it was assumed that because aggressive males fight more frequently (Bolhuis et al. 2005; Camerlink et al. 2015), they decrease investment in any single contest to avoid risks and contest costs. Thus, aggressive males may abandon a contest quickly even if they initiate them more frequently. Importantly and as noted above, aggressiveness does not predict fight outcomes in boar (Camerlink et al. 2015). The difference between young and old males is a clear indication that behavioral associations can change over time and context (cf. Briffa et al. 2015). However, relatively few studies have reported the disappearance of a behavioral correlation with age, so the generality (or not) of this pattern, is not clear.
There is more clarity on the effects of age on the likelihood of winning fights. In the brown butterfly Melanitis leda, male winning rates decrease with age (Kemp 2003), suggesting resource holding potential decreases with age in this species. On the other hand, in the northern elephant seal, older males win more contests, indicating that male resource holding potential increased with age (Haley et al. 1994), possibly due to positive effects of accumulated contest experience with aging. Indeed, many previous studies could not separate the effects of experience from aging per se (see Kemp 2002). Previous work on flour beetles (Okada et al. 2020), however, found older naive males with no previous fighting experience won more fights. Similarly, as males in our study had not engaged in fighting before, the age effects we find are not confounded by experience. Further studies are needed to disentangle experience and age effects on fighting behavior, and their role in shaping variation in age effects among species.
We found effects of genotype on aggression (fight latency) at young age, with genotype explaining about a fifth of the variation in aggression in young males. Other studies have also found direct and indirect genetic effects on elements of fighting behavior (e.g., Lane et al. 2020). However, the genetic effect we documented was not evident at older age, and in neither age classes was there a genetic effect on fight duration. Again, this reflects findings reported elsewhere (e.g., Simon et al. 2006; Shefferson et al. 2017; Lane et al. 2020), and studies in many systems find changes in genetic contributions to phenotypic variation with age (e.g., Coulson et al. 2006; Nussey et al. 2008). It is interesting that in an experimental evolution study of loser effects, fighting success did not evolve (Okada et al. 2019), which is consistent with our finding of limited genetic variation in fighting behavior. Additionally, the lack of genetic variation in fight duration at all ages means that the two behaviors we measure here (duration and latency) are not genetically correlated (or only very weakly so). Finally, we found no evidence for genetic trade-offs between early- and late-age performance, which again, is not without precedent (Nussey et al. 2008), but we note again there was a phenotypic association between aggression and fight duration at young age.
In summary, we find limited genetic variation for fighting behavior, and the significant genetic effect we did detect disappeared with age. Additionally, there were only effects of age on the duration of fighting, with male aggression being similar in young and old males. These findings are not in line with general theoretical predictions (e.g., Kemp 2006) of significant general effects of age coupled with specific effects of age on other male sexually-selected characters (e.g., Tidière et al. 2017). Future work should focus on disentangling the effects of aging and experience, to gain a more general view of the effects of age on fighting behavior in this and other species.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Andersson M (1994) Sexual selection. Sexual selection. Princeton University Press
Arking R (1998) Biology of Aging: observations and principles. Oxford University Press
Austad SN (1983) A game theoretical interpretation of male combat in the bowl and doily spider (Frontinella pyramitela). Anim Behav 31:59–73
Batchelor TP, Briffa M (2010) Influences on resource-holding potential during dangerous group contests between wood ants. Anim Behav 80:443–449
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Scheipl F, Grothendieck G (2015) Linear mixed-effects Modelsusing'Eigen'andS4. Package‘lme4’
Benus RF, Bohus B, Koolhaas JM, Van Oortmerssen GA (1991) Behavioural differences between artificially selected aggressive and non-aggressive mice: response to apomorphine. Behav Brain Res 43:203–208
Bolhuis JE, Schouten WG, Schrama JW, Wiegant VM (2005) Individual coping characteristics, aggressiveness and fighting strategies in pigs. Anim Behav 69:1085–1091
Bouwhuis S, Vedder O (2017) Avian escape artists? Patterns, processes and costs of senescence in wild birds. In The Evolution of Senescence in the Tree of Life (Eds.) RP Shefferson, OR Jones, R Salguero-Gómez, Cambridge University Press. pp. 156–174
Brandt Y (1999) When size is noteverything:determining the relative importance of two asymmetries influencing contest outcome. Anim Behav 57:F13–F14
Briffa M, Elwood RW (2005) Rapid change in energy status in fighting animals: causes and effects of strategic decisions. Anim Behav 70:119–124
Briffa M, Sneddon LU, Wilson AJ (2015) Animal personality as a cause and consequence of contest behaviour. Biol Lett 11 https://doi.org/10.1098/rsbl.2014.1007
Butler (2022) ASReml-ReferenceManual Version4. ASRemlestimatesvariancecomponentsunderageneral linear mixedmodelbyresidualmaximumlikelihood(REML). VSN International Ltd. UK.
Camerlink I, Turner SP, Farish M, Arnott G (2015) Aggressiveness as a component of fighting ability in pigs using a game-theoretical framework. Anim Behav 108:183–191
Candolin U (1998) Reproduction under predation risk and the trade–off between current and future reproduction in the threespine stickleback. Proc R Soc B 265:1171–1175
Coulson MW, Bradbury JR, Bentzen P (2006) Temporal genetic differentiation: continuous v. discontinuous spawning runs in anadromous rainbow smelt Osmerus mordax (Mitchill). J Fish Biol 69:209–216
Curio E (1983) Why de young birds reproduce less well? IBIS 125:400–404
Darwin C (1871) The descent of man, and selection in relation to sex. John Murry, London
David JR, Gilbert P, Legout H, Pétavy G, Capy P, Moreteau B (2005) Isofemale lines in Drosophila: an empirical approach to quantitative trait analysis in natural populations. Heredity 94:3–12
de Boer SFB, van der Vegt J, Koolhaas JM (2003) Individual variation in aggression of feral rodent strains: a standard for the genetics of aggression and violence? Behav Genet 33:485–501
Emlen DJ (2008) The evolution of animal weapons. Annu Rev Ecol Evol Syst 39:387–413
Glass C, Huntingford F (1988) Initiation and resolution of fights between swimming crabs (Liocarcinus Depurator). Ethology 77:237–249
Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E (2005) Functional senescence in Drosophila melanogaster. Ageing Res Rev 4:372–397
Haley MP, Deutsch CJ, Le Boeuf BJ (1994) Size, dominance and copulatory success in male northern elephant seals, Mirounga angustirostris. Anim Behav 48:1249–1260
Hillesheim E, Stearns SC (1992) Correlated responses in life-history traits to artificial selection for body weight in Drosophila melanogaster. Evolution 46:745–752
Hoffmann A, Parsons P (1989) Selection for increased desiccation resistance in Drosophila melanogaster: additive genetic control and correlated responses for other stresses. Genetics 122:837–845
Hosken DJ, Wilson AJ (2019) The problem of measuring trait-preference correlations without disrupting them. Behav Ecol 30:1518–1521
Humphries EL, Hebblethwaite AJ, Batchelor TP, Hardy ICW (2006) The importance of valuing resources: host weight and contender age as determinants of parasitoid wasp contest outcomes. Anim Behav 72:891–898
Ingleby FC, Hunt J, Hosken DJ (2013) Genotype-by-environment interactions for female mate choice of male cuticular hydrocarbons in Drosophila simulans. PLOS ONE. https://doi.org/10.1371/journal.pone.0067623
Jennings DJ, Hayden TJ, Gammell MP (2013) Personality and predictability in fallow deer fighting behaviour: the relationship with mating success. Anim Behav 86:1041–1047
Jonart LM, Hill GE, Badyaev AV (2007) Fighting ability and motivation: determinants of dominance and contest strategies in females of a passerine bird. Anim Behav 74:1675–1681.
Kelly CD, Godin JGJ (2001) Predation risk reduces male-male sexual competition in the Trinidadian guppy (Poecilia reticulata). Behav Ecol Sociobiol 51:95–100
Kemp DJ (2000) Contest behavior in territorial male butterflies: does size matter? Behav Ecol 11:591–596
Kemp DJ (2002) Sexual selection constrained by life history in a butterfly. Proce R Soc B 269:1341–1345
Kemp DJ (2003) Twilight fighting in the evening brown butterfly, Melanitis leda (L.) (Nymphalidae): age and residency effects. Behav Ecol Sociobiol 54:7–13
Kemp DJ (2006) Ageing, reproductive value, and the evolution of lifetime fighting behaviour. Biol J Linn Soc 88:565–578
Lane SM, Wilson AJ, Briffa M (2020) Analysis of direct and indirect genetic effects in fighting sea anemones. Behav Ecol 31:540–547
Lee VE, Head ML, Carter MJ, Royle N (2014) Effects of age and experience on contest behavior in the burying beetle, Nicrophorus Vespilloides Behav Ecol 25:172–179
Lee VE, Head ML, Carter MJ, Royle NJ (2013) Effects of age and experience on contest behavior in the burying beetle, Nicrophorus vespilloides. Behav Ecol 25:172–179
MacNulty DR, Smith DW, Vucetich JA, Mech D, Stahler DR, Packer C (2009) Predatory senescence in ageing wolves. Ecol Lett 12:1347–1356
Magnhagen C, Vestergaard K (1991) Risk taking in relation to reproductive investments and future reproductive opportunities: field experiments on nest-guarding common gobies, Pomatoschistus microps. Behav Ecol 2:351–359
Matsumura K, Abe MS, Sharma MD, Hosken DJ, Yoshii T, Miyatake T (2020) Genetic variation and phenotypic plasticity in circadian rhythms in an armed beetle, Gnatocerus cornutus (Tenebrionidae). Biol J Linn Soc 130:34–40
Maynard Smith J, Parker GA (1976) The logic of asymmetric contests. Anim Behav 24:159–175
Mowles SL, Cotton PA, Briffa M (2012) Consistent crustaceans: the identification of stable behavioural syndromes in hermit crabs. Behav Ecol Sociobiol 66:1087–1094
Nakagawa S, Johnson PCD, Schielzeth H (2017) The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14. https://doi.org/10.1098/rsif.2017.0213
Nussey D, Wilson A, Morris A, Pemberton J, Clutton-Brock T, Kruuk LEB (2008) Testing for genetic trade-offs between early- and late-life reproduction in a wild red deer population. Rroc R Soc B 275 https://doi.org/10.1098/rspb.2007.0986
Okada K, Katsuki M, Kiyose K, Okada Y (2020) Older males are more competitive in male fights and more aggressive toward females in the broad-horned flour beetle Gnatocerus Cornutus. Behav Ecol Sociobiol 74:1–10
Okada K, Miyanoshita A, Miyatake T (2006) Intra-sexual dimorphism in male mandibles and male aggressive behavior in the broad-horned flour beetle Gnatocerus Cornutus (Coleoptera: Tenebrionidae). J Insect Behav 19:457–467
Okada K, Miyatake T (2009) Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatocerus Cornutus. Anim Behav 77:1057–1065
Okada K, Miyatake T (2010) Effect of losing on male fights of broad-horned flour beetle, Gnatocerus Cornutus. Behav Ecol Sociobiol 64:361–369
Okada Y, Katsuki M, Okamoto N, Fujioka H, Okada K (2019) Fighting ability and motivation: determinants of dominance and contest strategies in females of a passerine bird. PLoS Biol 27. https://doi.org/10.1371/journal.pbio.3000541
Parker GA (1974) Assessment strategy and the evolution of fighting behaviour. J Theor Biol 47:223–243
Parker GA, Maynard Smith J (1990) Optimality theory in evolutionary biology. Nature 348:27–33
Poizat G, Rosecchi E, Crivelli AJ (1999) Empirical evidence of a trade–off between reproductive effort and expectation of future reproduction in female three-spined sticklebacks. Proc R Soc B 266:1543–1548
Prenter J, Elwood RW, Taylor PW (2006) Self-assessment by males during energetically costly contests over precopula females in amphipods. Anim Behav 72:861–868
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
R Core Team (2017) R: A Language and Environment for Statistical Computing. https://www.R-project.org/
Rose MR (1991) Evolutionary Biology of Aging. Oxford University Press on Demand
Self SG, Liang KY (1987) Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. Am Stat Assoc 82:605–610
Shefferson RP, Jones OR, Salguero-Gómez R (2017) The evolution of Senescence in the Tree of Life. Cambridge University Press
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. TREE 19:372–378
Simon AF, Liang DT, Krantz DE (2006) Differential decline in behavioral performance of Drosophila melanogaster with age. Mech Ageing Dev 127:647–651
Stearns SC (1992) The evolution of life histories. Oxford University Press
Taylor PW, Elwood RW (2003) The mismeasure of animal contests. Anim Behav 65:1195–1202
Taylor PW, Hasson O, Clark DL (2001) Initiation and resolution of jumping spider contests: roles for size, proximity, and early detection of rivals. Behav Ecol Sociobiol 50:403–413
Thornhill R, Alcock J (2013) The evolution of insect mating systems. Harvard University Press
Tidière M, Lemaître JF, Pélabon C, Gimenez O, Gaillard JM (2017) Evolutionary allometry reveals a shift in selection pressure on male horn size. 30:1826–1835
Acknowledgements
This work was supported by Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Fellows to KM (no. 20J00383) and a grant from the Japan Society for the Promotion of Science KAKENHI 21H02568 and 21K19116 to TM.
Funding
Open Access funding provided by Okayama University.
Author information
Authors and Affiliations
Contributions
KM, MD and TM conceived and designed the study. TN and KM performed the experiment and KM, EP and DJH data analyses. KM, TM, EP and DJH contributed to the interpretation of data, and drafted the manuscript. All authors approved the final version and agree to be accountable for the content therein.
Corresponding author
Ethics declarations
Competing interests
We declare that we have no competing interests.
Additional information
Communicated by A. Toth.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nishitani, T., Matsumura, K., Postma, E. et al. Experimental quantification of genetic and ontogenetic effects on fighting behavior in the broad-horned flour beetle. Behav Ecol Sociobiol 78, 34 (2024). https://doi.org/10.1007/s00265-024-03451-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03451-w