Abstract
While there are many studies documenting female mating preferences across taxa, male mate choice remains relatively understudied. Male mate choice often develops when there is variation in female quality and thus the fitness benefits of mating with particular females. Specifically, males tend to prefer females with traits that confer direct fitness benefits such as large body size, which may be linked with high fecundity. Prior work has shown that females of the strawberry poison frog, Oophaga pumilio, prefer males bearing certain coloration (most often the female’s own color), and that this preference can be learned through maternal imprinting. Females have been shown to prefer larger males as well. Here we test whether similar mate preferences for color and size exist in males of this species using two-way choice tests on captive bred male O. pumilio. In each test focal males were placed in an arena with two stimulus females: either both of the same size but differing in color, or both of the same color but differing in size. We found only weak evidence for behavioral biases toward particular colors and no evidence for biases toward larger females, suggesting that males of O. pumilio do not predictably choose mates based on these female traits. Despite several aspects of their natural history that suggest males have reasons to be choosy, our findings suggest that the cost of mate rejection may outweigh any fitness benefits derived from being selective of mates. Studies of additional populations, ideally conducted on wild individuals, are needed to better understand the range of conditions under which males may exhibit mate choice and the types of traits on which they base these choices.
Significance statement
To fully understand the fitness landscapes and evolutionary trajectories that result from sexual selection, we need to understand when and how the mate preferences of the two sexes act and interact. While female mate choice has been widely studied, male mate choice remains poorly understood. To help bridge this gap, we studied male mate preferences in the strawberry poison frog Oophaga pumilio, a small brightly colored frog for which female preferences for male color and size have been well-documented. We found no evidence that male O. pumilio exhibit mate preferences based on female size and little evidence for male mate preferences based on female color. This is surprising given that larger females are often more fecund, male O. pumilio are known to exhibit color-based behavioral biases in the context of male-male competition, and both sexes provide parental care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male mate choice occurs when males show a preference for females with specific traits. This can affect the evolution of these traits in females or the population as a whole, thus influencing the fitness of future generations (Edward and Chapman 2011). The evolutionary implications of male mate choice differ from those of female choice; males tend to prefer traits that provide direct fitness benefits (i.e., improvements to their own survival or reproduction, such as indicators of female reproductive status) as opposed to indirect benefits (i.e., improvements to the fitness of relatives, such as increased offspring survival; Nakahashi 2008). While there are many studies that investigate and discuss the significance of female mate choice, very few of these consider the potential evolutionary implications that male mate choice may have within the same species. In many species in which female choice has been studied, male choice has not. Moreover, most of the examples that have documented male mate choice come from arthropods (Tuschhoff and Wiens 2023), highlighting the need for further study of this form of mate choice in other animal phyla. Additionally, for many species known to exhibit both male and female mate choice, such as Drosophila melanogaster (fruit fly), Poecilia reticulata (guppy), and Xiphophorus hellerii (swordtail fish), female choice has been much better studied (Dosen and Montgomerie 2004; Byrne and Rice 2006; Tudor and Morris 2008). Thus, while numerous studies have detailed the effects of female mate choice on the evolution of populations and species, the same emphasis has not been historically placed on determining whether or how much male choice may also be impacting evolutionary trajectories. More attention toward discovering and understanding instances of male mate preferences may help elucidate complexities of mating behavior and mechanisms of sexual selection and evolution that would be missed by focusing on female choice alone.
Male mate choice tends to evolve under certain conditions and life histories. First, there must be variation in the quality of females and the associated fitness benefits males receive by mating with them. Male mate choice is more likely to develop if this variation in quality is linked to direct fitness benefits to the male. On one hand, female quality may be related to body size, such as when larger females have higher fecundity (Edward and Chapman 2011). On the other hand, female quality may be related to coloration, such as when coloration is indicative of reproductive state (Amundsen and Forsgren 2001), indicative of higher fitness (Baldauf et al. 2011), or prevents costly heterospecific courting and mating (Shahandeh et al. 2018). Moreover, the development of male mate choice is influenced by the number of females available for mating and is favored when the cost of mate rejection is low (i.e., many receptive mates are available and/or investment in courtship is low) and the probability of finding another mate is high (Johnstone et al. 1996). As a result, male mate choice is more likely to evolve when many receptive females are available and/or in mating systems where males encounter multiple females at once, rather than consecutively (Barry and Kokko 2010). Female choice can also influence male mate choice, as attractive males have a higher potential to mate (and choose mates) than unattractive males. This could cause male mate choice to become condition-dependent, with increased mating between males and females of similar quality. One example of this is seen in the two-spotted goby (Gobiusculus flavescens), in which higher quality, larger males show stronger preferences toward more desirable, colorful females than do smaller males (Amundsen and Forsgren 2003). Interestingly, in situations where males face greater competition as a result of preferring the same female, male mate choice may even lead to the development of alternative preferences for different trait values. This has been shown in the orb-weaving spider (Zygiella x-notata), which exhibits size assortative mating when sexual competition is strong (Bel-Venner et al. 2007).
It is theorized that males and females of most animal species have conventional sex roles based on the Darwin-Bateman paradigm, in which parental care is more common in females, and sexual dimorphism and stronger sexual selection is more common in males (Janicke et al. 2016). Accordingly, male mate choice and female-female competition tend to be observed in species with “reversed” or non-conventional sex roles, where males contribute more to parental care than females and are unable to mate with all available females. This reversal favors males who assess female quality and preferentially mate with those with desired traits (Gwynne 1991). For example, male pipefish (Syngnathus typhle) invest more effort in parental care than females and thus are the choosier sex. As a result, intrasexual competition is more intense among females than males, and male mate choice significantly impacts offspring fitness (Sandvik et al. 2000). However, male mate choice exists in some species where males do not contribute much to parental care. This is especially true in species where males invest more resources into mating effort (e.g., attracting and competing for females; Edward and Chapman 2011). For example, two-spotted gobies (Gobiusculus flavescens; Amundsen and Forsgren 2001), spiny-footed lizards (Acanthodactylus erythrurus; Belliure et al. 2018), and the fruit fly (D. melanogaster; Byrne and Rice 2006) are all species with conventional sex roles and low male investment that still display male mate choice. This is significant, as these “conventional” species tend to show female choice as well, and the interplay between male and female choice in such species is often understudied.
In anurans, like many other taxa, female choice is much better studied than male choice. In most frog species that display female choice, female preferences are based on variation in male acoustic signals (Burmeister 2017). These preferences are well-documented across frog species (Gerhardt et al. 2000; Ron 2008), including poison frogs (Dreher and Pröhl 2014), and the neurobiological mechanisms behind these behaviors have been studied in depth (Burmeister 2017). Female mate choice based on visual signals, such as color (e.g., Summers et al. 1999) or body size (e.g., Swierk and Langkilde 2021), and multimodal signals (e.g., acoustic signals coupled with visual ones as in de Luna et al. 2010) have also been documented in a number of frog species. Nevertheless, when it comes to male mate choice in frogs, the literature is sparse. One frog species known to display male mate choice is the wood frog (Rana sylvatica). Male wood frogs preferentially mate with females of similar size as themselves, as size mismatch severely reduces fertilization (Swierk and Langkilde 2021). Thus, selecting mates based on size provides direct fitness benefits that likely outweigh the cost of mate rejection, especially as this species exhibits an explosive breeding system in which many receptive females are available simultaneously (Berven 1981). Male mate choice has also been documented in another explosive breeder Rana kukanoris, in which males prefer larger females due to their higher fecundity (Chen and Lu 2011). Interestingly, there is also evidence of intra-female competition in the green poison dart frog, Dendrobates auratus. While females are still more selective than males in this species, they compete with other females to prevent them from mating with specific males in order to secure high quality males for parental care (Summers 1989). While male mate choice has not been studied in this species, the species does meet some of the criteria for the evolution of male mate choice as males invest significant energy into parental care of offspring and may be limited in their ability to mate with many females due to mate guarding by female partners (Summers 1990).
Here we test for the presence of male mate choice in strawberry poison frogs, Oophaga pumilio. These small (snout-vent length (SVL) from 1 to 2 cm; Siddiqi et al. 2004), brightly colored frogs show extreme variation in coloration across populations found in the Bocas del Toro region of Panama but little to no sexual dichromatism (i.e., both males and females are similarly brightly colored; Summers et al. 2003; but see Maan and Cummings 2009). While many studies have demonstrated female choice based on male color (Summers et al. 1999; Reynolds and Fitzpatrick 2007; Maan and Cummings 2008, 2009; Richards-Zawacki and Cummings 2011; Dreher and Pröhl 2014; Gade 2015; Gade et al. 2016; Yang et al. 2016, 2019a, b) and size (Gade 2015; Gade et al. 2016), to date there have been no published studies on male mate choice in this species. Nonetheless, the reproductive roles and constraints on male O. pumilio suggest the potential for the evolution of behavioral biases.
O. pumilio has a promiscuous mating system, in which both males and females will court and mate multiple times with different partners (Pröhl and Hödl 1999). Promiscuous mating systems may promote the evolution of mate choice by facilitating the encounter of preferred traits in potential partners while reducing the cost of rejecting low-quality mates (Peignier et al. 2022). Frogs of this species also provide biparental care to offspring in which the male tends the eggs, and the female cares for the tadpoles after they hatch. However, this parental care behavior is asymmetrical, as the female’s role requires more time and energy than the male’s (Dugas 2018). Behavioral biases may evolve in the sex that provides less care as this sex gains the greatest direct benefit from choosing a suitable offspring-caring partner (Johnstone et al. 1996). Additionally, although males are usually the territorial and aggressive sex, female territoriality and aggression between females has also been documented in O. pumilio (Haase and Pröhl 2002; Meuche et al. 2011). Males also aggregate close to females’ territories, which in turn, are spatially clustered close to suitable tadpole rearing sites (Pröhl and Berke 2001). Therefore, given that encountering multiple females is feasible, the evolution of male choosiness may be facilitated by the low cost of searching for a preferred mate. Finally, it has previously been established that O. pumilio tadpoles imprint on the coloration of their mother, and that female O. pumilio grow up to prefer males of the mother’s coloration. Likewise, imprinting leads to males that are more aggressive toward other males of their mother’s coloration (Yang et al. 2019a). This result suggests that color discrimination may act as a reproductive barrier between diverging populations and indicates that behavioral biases based on coloration have indeed evolved in males of O. pumilio. However, to date, such color-associated biases have only been investigated in males in the context of aggressive behaviors. Here, we test the hypotheses that males show preferences for (1) large females (which are typically more fecund; Donnelly 1989) and (2) females of the same color as themselves (as such color-associated biases are seen in female mate choice and male aggression in this species; Yang et al. 2019a). To do this, we used two-way choice tests on frogs from a laboratory colony to quantify male mating preferences based on female coloration and body size. Our findings contribute to an improved understanding of the evolution of mating preferences and associated traits in this species, as well as the factors that promote or hinder the evolution of male mate choice more broadly.
Materials and methods
Study system
For our study, we used O. pumilio individuals from a captive colony housed at the University of Pittsburgh. All focal males and all but a few of the stimulus females (which were wild-caught in Panama in 2009) we used were lab-bred, and all frogs have been maintained in captivity in accordance with the methods described in Dugas and Richards-Zawacki (2015). Due to the limited availability of virgin males, many of the males used in this experiment came from same-morph breeding pairs (i.e., where one male and one female of the same morph had been housed together in breeding enclosures). We used individuals from two differently colored populations: the Popa (green) color morph, captive-bred from wild-caught individuals from the Punta Laurel population of Isla Popa in the Bocas del Toro region of Panama, and the Bastimentos West (red) color morph, captive-bred from wild-caught individuals from the population at Cemetery Hill, Isla Bastimentos population in Bocas del Toro. Both morphs are indicated in purple on the map in Fig. 1. Both the Popa (green) and Bastimentos West (red and yellow polymorphic) populations are known to exhibit color-assortative female mate choice (Richards-Zawacki and Cummings 2011; Yang et al. 2019a) and male-male competition (Yang et al. 2019a; MG-S et al. unpublished data), but male mate choice has not been studied in either.
Map showing the locations of different color morphs of O. pumilio in the Bocas del Toro Archipelago of Panama (large map) and the location of the Archipelago with respect to the species range (inset, top right). The focal populations included in our study are labelled in bold and colored in purple on the map and images of these color morphs are shown bracketed in purple on the right. Populations labeled with stars are polymorphic and images of the morphs found in sympatry at these sites are shown at the lower right. Photographs by V. Prémel, J.P. Lawrence, S.A. Echeverri, I.J. Wang, and Y. Yang
We housed focal males individually in an enclosure with black opaque walls for ~ 48 h prior to behavioral assays. We did this because in prior experiments we have found time away from their prior social setting to improve the odds of individuals exhibiting courtship behaviors during trials. Immediately prior to each trial, we measured the SVL of each focal male to the nearest 0.1 mm using dial calipers. We conducted this study in two experiments with similar methodologies: the first part tested for male mate preferences based on female coloration, and the second tested for male mate preferences based on female size.
Experiment 1: color
We conducted the color portion of the study using 37 male O. pumilio as focal individuals. Of these, 21 were of the Popa (green) color morph, and the other 16 were of the Bastimentos West (red) color morph. These color morphs were chosen because they are visually distinct in coloration. We removed 4 of those trials (1 Bastimentos West red and 3 Popa green) due to prior co-housing of the focal male and one of the stimulus females, giving a final sample size of 33 consisting of 18 Popa green and 15 Bastimentos West red. We tested more Popa males than Bastimentos West due to limited availability of adult male Bastimentos West individuals in our captive colony. Pairs of opposite-morph (i.e., one red, one green), size-matched female O. pumilio were used as stimulus individuals for the color choice assays. For each trial, we selected one pair of stimulus females at random from a set of size-matched pairs.
Experiment 2: size
For the size choice study, we used 30 Popa (green) males, but removed 3 of those trials from analysis due to prior co-housing of the focal male with one of the stimulus females (final n = 27). We chose this because similar or smaller sample sizes have been sufficient to detect significant mate preferences in this species in previous studies (see Table S1). We used pairs of Popa (green) females differing by at least 2.0 mm (~ 10–15%) in SVL as stimulus individuals and selected these pairs at random from a set of such size-distinct pairs prior to each trial. We used SVL as our size parameter because it can be measured quickly (reduces handling time and stress on the frog). Bastimentos West (red) frogs were not used as focal or stimulus individuals in this experiment due to sample size limitations.
Behavioral assays
We conducted behavioral assays in a 30 × 40 × 20 cm plastic arena containing sheet moss as a substrate, which was moistened with water prior to each set of trials. The top of the arena was left open during the trials, but a plastic mesh around the edges prevented frogs from escaping. We conducted the assays in a dark room maintained at 23–25 °C and with a lighting setup designed to simulate the conditions of the forest floor (following Maan and Cummings 2008). The behavioral assay protocol followed the setup in Yang et al. (2019a) but with the sexes of focal and stimulus frogs reversed (Fig. 2).
Experimental setup of the two-way choice tests. The trials were conducted in a 30 × 40 × 20 cm arena, and stimulus females were placed under transparent plastic domes equidistant from the center. Each stimulus dome was placed in the center of a 4 cm “interaction zone,” and the stimulus male was allowed to roam freely in the arena during the trials
During the trials, we confined the stimulus females under small, transparent plastic domes equidistant from the center of the arena (Fig. 2). Each dome was placed in the center of a plastic disk that denoted the 4 cm “interaction zone” surrounding the dome. We allowed the stimulus females to acclimate to the domes for 15 min prior to the addition of the focal male frog to the arena. Upon adding the focal male, we initially confined him under a larger transparent plastic dome, which was attached overhead to a simple pulley system. We immediately placed an opaque black plastic cover over the focal male’s transparent dome and allowed him 5 min to acclimate to this new environment. After five min, we lifted the black cover but not the transparent dome, and allowed the male another two minutes to view the stimulus females from within the clear dome. After the two min, we lifted the transparent dome, so the focal male could move freely in the arena.
Data were recorded by an unblinded observer (i.e., a person who could readily assess the color and body size of the focal and stimulus animals) via direct observation between the hours of 8:44 and 16:50. While male O. pumilio are most reproductively active (call most intensely) between 6:00 and 10:00 in the field (Bunnell 1973), frogs in our colony have been observed to call frequently during all daylight hours (6:00–18:00). The first half of each trial lasted 15 min, and the trial began when the first interaction between the male and a female (defined as the focal male entering the 4 cm “interaction zone” of either stimulus female) commenced. If the male failed to enter either female’s interaction zone within the first 30 min, we considered the trial “failed,” and we tested the focal male again on a different day. To account for potential side biases, after the first half of the trial we once again confined the focal male under the plastic dome and black cover and switched the positions of the stimulus females. We similarly allowed the male five minutes of dark acclimation and two minutes of time to view the females from within the clear dome before a second 15-min period where the male was allowed to move freely in the arena.
Behaviors of interest
During each behavioral assay, we recorded several behaviors indicative of the focal male’s interest in a female, which we based on observations of courtship and mating in wild O. pumilio (Yang et al. 2019b). We recorded the number of approaches toward each of the stimulus females with an approach being defined as the focal male orienting towards and entering the interaction zone of a stimulus female. We also measured the length of time the focal male spent in the interaction zone for each approach, as well as the total association time spent with each female over the course of the trial. The number and duration of calling behavior by the focal male was also recorded, with one call being defined as the male orienting towards a female and initiating calling after a period of silence (at least 3 s). Finally, we quantified the number of times the male climbed on the female’s dome (or touched it with at least two feet while oriented towards the female). Calling and climbing behavior did not occur with enough frequency to be useful metrics of mating interest and were therefore not used in our statistical analyses.
Statistical analysis
Color biases
We quantified association time for the color experiment as the proportion of time spent with the stimulus female of the same color as the male divided by the total association time spent with both females. We similarly quantified the proportion of approaches. We analyzed the proportion of association time and proportion of approaches toward females of the same morph as the focal male. To avoid issues with normality assumptions for proportional data we used directed, one-sample permutational t-tests (with γ/α = 0.8 and δ/𝛾 = 0.25, following Rice and Gaines 1994), comparing the data to the null hypothesis of a population mean of ≤ 0.5 (no preference for own-colored female). For each of these metrics, values > 0.5 indicate a behavioral bias in the direction that would support the alternative hypothesis: that males show preferences for own-colored females. We analyzed each metric independently for each morph (Popa green and Bastimentos West red). Because sample sizes per morph were small, we also ran the permutational t-test with both morphs together to investigate the potential for low statistical power causing us to fail to reject the null hypothesis.
Size biases
We quantified association time for the female size experiment as the proportion of time spent with the larger stimulus female divided by the total association time spent with both females. We similarly quantified the proportion of approaches. We analyzed the proportion of association time and proportion of approaches to the larger female in the same way as in the color bias experiment, using directed, one-sample permutational t-tests (with γ/α = 0.8 and δ/𝛾 = 0.25, following Rice and Gaines 1994) to test for proportions greater than the null hypothesis of ≤ 0.5 (no preference for larger females). In this case, only the Popa (green) population was examined.
We used the function perm.test (package jmuOutlier) to perform the t-tests and the function cohensD (package lsr) to find effect sizes. All statistical analyses were conducted in R using RStudio version 4.3.1 (RStudio Team 2019; R Core Team 2020).
Results
Color assays
We conducted 55 trials using our 37 focal individuals. Of these, 18 trials were considered “failed” (focal male did not reach the interaction zone within 30 min of observation) and 37 were successful; however, 4 of these successful trials (3 Popa green and 1 Bastimentos West red) were removed from analysis due to prior co-housing of the focal male and one of the stimulus females. When considering both color morphs together, we did not find support for male behavioral biases based on female coloration. Neither the proportion of association time (Table 1) nor the proportion of approaches (Table 2) were significantly greater than 0.5. When considering each morph independently, the proportion of association time for the Popa population was nearly significantly greater than 0.5 (Table 1, P = 0.068) but we did not find support for an assortative color preference in this metric for the Bastimentos West population (Fig. 3). The proportion of approaches was not significantly greater than 0.5 regardless of whether we considered both populations together or each separately (Fig. 4, Table 2).
Proportion of time that males of each population spent with a female of their own morph. A proportion significantly greater than 0.5 (dashed line) indicates a male preference towards same-colored females. Bold lines indicate medians. Boxes enclose 25th to 75th percentiles. Dots are data points. Error bars enclose data range, excluding any outliers (defined as dots appearing above or below error bars). Colors denote the typical population coloration (red for Bastimentos West and green for Popa)
Proportion of approaches of males of each population towards a female of their own morph. A proportion significantly greater than 0.5 (dashed line) indicates a male preference towards same-colored females. Bold lines indicate medians. Boxes enclose 25th to 75th percentiles. Dots are data points. Error bars enclose data range, excluding any outliers (defined as dots appearing above or below error bars). Colors denote the typical population coloration (red for Bastimentos West and green for Popa)
Size assays
We conducted 52 trials using our 30 focal individuals. Of these, 22 trials were considered “failed” and 30 were considered successful; however, 3 of these successful trials were removed from analysis due to prior co-housing of the focal male and one of the stimulus females (final n = 27). We did not find support for male behavioral biases based on female size. This lack of support was evident for both the proportion of association time (Fig. 5, Table 1) and the proportion of approaches (Fig. 5, Table 2), which were not significantly greater than 0.5.
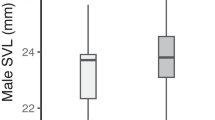
Proportion of approaches and time spent of Popa green males towards a female of their own morph. A proportion significantly greater than 0.5 (dashed line) indicates a male preference towards larger females. Bold lines indicate medians. Boxes enclose 25th to 75th percentiles. Dots are data points. Error bars enclose data range, excluding any outliers (defined as dots appearing above or below error bars)
Discussion
We used two-way choice tests to test whether male strawberry poison frogs (O. pumilio) demonstrate preferences for larger females and/or females of the same color as themselves. Our results suggest that, unlike females (see Tables S1 and S2) male O. pumilio do not show color- or size-assortative mate preferences. We predicted that males would show some preference for at least one of these female traits for several reasons: courtship is both lengthy and intensive (lasting on average 1.5 h; Pröhl and Hödl 1999), both males and females contribute to parental care (Pröhl and Hödl 1999), this species has a promiscuous mating system (Pröhl and Hödl 1999), larger females are more fecund, thus conferring more direct fitness benefits (Donnelly 1989), and males show aggressive behavioral biases toward other males based on coloration (Yang et al. 2019a). However, we found little evidence for male mate choice based on female size or color. Only for the Popa population did one metric, the proportion of time spent interacting with females of the local green phenotype, exhibit a pattern that could have been interpreted as a marginally significant assortative mate preference. Given this ambiguous result, we discuss our findings in light of sexual selection theory and the sexes' differential reproductive roles.
Although both males and females contribute to parental care, female O. pumilio invest more time and energy into parental care than males. Females are responsible for transporting the tadpoles to their leaf axil nurseries after they hatch and feeding them unfertilized eggs until they complete metamorphosis (Pröhl and Hödl 1999). This means that the potential reproductive rate (PRR) is higher for males while females are the limiting reproductive resource (i.e., females have a longer reproductive “time-out”; Pröhl 2002). This imbalance in parental care may explain why females often show significant mating preferences in this species while males in the current study generally did not. For males, the benefits of mating indiscriminately may outweigh the costs of courting and engaging in parental care with low quality females. This idea is supported by a meta-analysis for all animals, including Allobates femoralis, a related species of dendrobatoid frog (the poison frog superfamily containing O. pumilio), which found a steeper Bateman gradient (i.e., a larger increase in fitness with each additional mating) for males of most species (including A. femoralis) in comparison to females (Ursprung et al. 2011; Janicke et al. 2018). In such cases, males are predicted to increase their fitness more by mating with many partners and focusing on offspring quantity rather than quality, thus disfavoring the evolution of male mate choice.
Another potential explanation for the lack of male choice in O. pumilio may be related to how often males encounter receptive females in the wild. For example, a study on the sexually cannibalistic praying mantid Pseudomantis albofimbriata showed that even in species with significant variation in female quality, if males encounter females sequentially (rather than simultaneously), this may prevent the evolution of male mate choice. This is because the fitness cost of rejecting a potential mate is high if the male is unlikely to find another mate, making it more beneficial for him to mate with all available females, regardless of quality, rather than being selective (Barry and Kokko 2010). Likewise, O. pumilio males are likely to encounter females sequentially, as they court females within small non-overlapping territories and receptive females approach calling males prior to courtship (Pröhl and Hödl 1999). Moreover, while female O. pumilio have larger home ranges than males, these are still small enough that a male’s territory may only overlap with the home ranges of a few females, only some of whom may be receptive to mating at any given time. Females are not receptive when they are caring for tadpoles because they use their own unfertilized eggs to feed the tadpoles (Pröhl and Hödl 1999). This would serve to further limit the number of receptive mates a male may encounter within a given time frame and further supports the idea that sequential encounters may also be acting to hinder the evolution of male mate choice in this species.
Additionally, our study was conducted using captive-reared individuals in a laboratory setting. While we adjusted the lighting, temperature, and humidity to emulate natural conditions during our experiments, it is possible our findings may have differed if the experiment were carried out with wild O. pumilio in the males’ natural territories. Male O. pumilio keep their territories for years and are familiar with the resources available within them, such as potential oviposition, calling, and defense sites (McVey et al. 1981; Pröhl and Hödl 1999). In light of this, males may be more motivated to pursue females (and potentially exert preferences) in their familiar wild habitats as opposed to the unfamiliar setting of a laboratory arena.
While the results of our study suggest that, at least under captive conditions, male O. pumilio may not be selective about the color or size of their mates, our study only tested two or one population(s), respectively, and was limited in sample size. Moreover, we used SVL as our factor of interest for the size experiment, but we cannot rule out that other indicators of size (i.e. mass or volume) may have the potential to influence mate preferences. For example, a previous study on O. pumilio found a significant female preference for males with greater mass (but did not measure SVL; Gade 2015). Additionally, we found variation among the two populations such that the difference in male interaction time with females of different colors approached statistical significance in one population (see Table 1, Popa “Green”) but not in the other. Similar variation in the strength of behavioral biases in the two sexes has been seen among O. pumilio populations in previous studies (e.g., Yang et al. 2016, 2018) suggesting that studies of male mate choice in additional populations could uncover clearer evidence for male mate choice. Differences in the operational sex ratio (i.e., the ratio of males to females that are ready to mate; Pröhl 2002), or habitat conditions that facilitate the recognition of preferred signals (e.g., light availability; Yang et al. 2019b), are known to affect the direction and intensity of sexual selection (Pröhl 2002) and likely vary among wild O. pumilio populations. However, since our study was performed using captive-bred individuals in a laboratory setting, we would not expect such differences in our study. It is also possible that male mate choice exists in our studied populations but that the biases are subtle enough (i.e., small enough effect size) that we could not detect them as significant without a larger sample size. However, the sample sizes that we used are on par with previous studies of O. pumilio that have found significant female mate preferences (see Tables S1 and S2). Our methodology differs from Yang et al. (2019a), which found significant female choice in lab-reared O. pumilio, in that our focal frogs were not naive to encounters with the opposite sex. In our study, all of the males we tested had been housed with opposite-sex individuals of the same and/or different colors as themselves prior to testing. Since O. pumilio are known to learn color-associated behavioral biases based on social interactions (i.e., with their mothers; Yang et al. 2019a), we cannot rule out the possibility that learning based on prior social experiences with females may have affected the behaviors we observed in this study as well. However, many previous studies on wild-caught (and presumably not naive) O. pumilio have documented significant female choice based on both male color and male size despite a presumed history of previous experience with the opposite sex (see Tables S1 and S2).
Imprinting does not influence male mate choice in the same way as male aggression
While we did not find support for our hypotheses regarding male mate choice, this study’s results do shed some additional light on the role that learning via imprinting plays in this species. Both sexes of O. pumilio are known to imprint on the color of their mother as tadpoles, but males use this color-associated learning to bias later life aggressive behaviors toward males of the same color as their mother, while females grow up to prefer mates of the same color as their mother (Yang et al. 2019a). It is interesting that while this learned color bias influences competitive decisions in male O. pumilio, it does not appear to carry over to bias male behaviors in the context of mate choice the way it does for females. Sex differences in imprintability have previously been documented in birds (ten Cate and Vos 1999), including the zebra finch (Taeniopygia guttata), a sexually dimorphic species with biparental care, similar to O. pumilio. Sex differences in imprinting in zebra finches have been attributed to the differing roles of males and females in courtship and appear to influence sexual selection and sex differentiation later in life, suggesting that these differences are important to mating success (Vos 1995). Additional research is needed to deepen our understanding of the mechanisms that produce these types of differences in learned behavioral biases between males and females.
Future directions
While O. pumilio do not appear to display color- or size-associated male mate preferences, there are many other species within the poison frog family Dendrobatidae that show a variety of parental care strategies, many of which are either male-biased or male-only. In fact, the male is the primary caregiver in most dendrobatid species (Carvajal-Castro et al. 2021). As male mate choice most commonly develops in species with reversed sex roles (Gwynne 1991), this form of sexual selection may potentially be widespread among poison frog species that exhibit male-dominated parental care and female-female competition, such as the green poison dart frog Dendrobates auratus (Summers 1989, 1990). Additionally, over 330 species within the neotropical poison frog superfamily Dendrobatoidea display sexual dichromatism, often in species that are cryptically colored rather than aposematic. In at least one such species, Colosthethus imbricolus, female reproductive fitness is correlated with the conspicuousness of her colorful markings, suggesting the potential for male mate choice in sexually dichromatic poison frog species (Palacios-Rodríguez et al. 2022). Hence, while we found no evidence for male mate choice in O. pumilio, it may have evolved in other poison frog species, and particularly in those with male-dominated parental care and/or forms of sexual dichromatism where females are more conspicuous. Future research into male mate choice in related species of poison frogs with different life histories could go a long way toward improving our understanding of the evolution of this understudied form of sexual selection.
The need to better understand the occurrence and importance of male mate choice, however, extends beyond poison frogs. A recent meta-analysis of large-scale patterns of sexual selection found that while female choice is the most common mechanism of sexual selection, 40% of animals displaying sexually selected traits demonstrate some form of male mate choice, and this is particularly prevalent in arthropods (Tuschhoff and Wiens 2023). Considering the historical bias towards studying female choice, especially in vertebrates, these data suggest that male mate choice may be more common within the animal kingdom than previously thought. As many species exhibiting male choice also exhibit female choice, a deeper analysis into male mate choice in species where only female choice has been studied would help us better understand the pattern of occurrence and potential for interactions between these two types of biases. While in some species, as appears to be true with O. pumilio, females may truly be the choosier sex, further study of male mate choice across taxa is needed to reject the null hypothesis that male mate choice is equally likely to evolve and contribute to shaping the patterns and effects of sexual selection in animals.
Data availability
All data used in this study is available as supplementary material.
References
Amundsen T, Forsgren E (2001) Male mate choice selects for female coloration in a fish. P Natl Acad Sci USA 98:13155–13160. https://doi.org/10.1073/pnas.211439298
Amundsen T, Forsgren E (2003) Male preference for colourful females affected by male size in a marine fish. Behav Ecol Sociobiol 54:55–64. https://doi.org/10.1007/s00265-003-0593-4
Baldauf SA, Bakker TCM, Kullmann H, Thünken T (2011) Female nuptial coloration and its adaptive significance in a mutual mate choice system. Behav Ecol 22:478–485. https://doi.org/10.1093/beheco/arq226
Barry KL, Kokko H (2010) Male mate choice: why sequential choice can make its evolution difficult. Anim Behav 80:163–169. https://doi.org/10.1016/j.anbehav.2010.04.020
Belliure J, Fresnillo B, Cuervo JJ (2018) Male mate choice based on female coloration in a lizard: the role of a juvenile trait. Behav Ecol 29:543–552. https://doi.org/10.1093/beheco/ary005
Bel-Venner MC, Dray S, Allainé D, Menu F, Venner S (2007) Unexpected male choosiness for mates in a spider. Proc R Soc Lond B 275:77–82. https://doi.org/10.1098/rspb.2007.1278
Berven KA (1981) Mate choice in the wood frog, Rana sylvatica. Evolution 35:707–722. https://doi.org/10.2307/2408242
Bunnell P (1973) Vocalizations in the territorial behavior of the frog Dendrobates pumilio. Copeia 1973:277–284. https://doi.org/10.2307/1442966
Burmeister SS (2017) Neurobiology of female mate choice in frogs: auditory filtering and valuation. Integr Comp Biol 57:857–864. https://doi.org/10.1093/icb/icx098
Byrne PG, Rice WR (2006) Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc R Soc Lond B 273:917–922. https://doi.org/10.1098/rspb.2005.3372
Carvajal-Castro JD, Vargas-Salinas F, Casas-Cardona S, Rojas B, Santos JC (2021) Aposematism facilitates the diversification of parental care strategies in poison frogs. Sci Rep 11:19047. https://doi.org/10.1038/s41598-021-97206-6
Chen W, Lu X (2011) Sex recognition and mate choice in male Rana kukunoris. Herpetol J 21:141–144
de Luna AG, Hödl W, Amézquita A (2010) Colour, size and movement as visual subcomponents in multimodal communication by the frog Allobates femoralis. Anim Behav 79:739–745. https://doi.org/10.1016/j.anbehav.2009.12.031
Donnelly MA (1989) Reproductive phenology and age structure of Dendrobates pumilio in northeastern Costa Rica. J Herpetol 23:362–367. https://doi.org/10.2307/1564047
Dosen LD, Montgomerie R (2004) Female size influences mate preferences of male guppies. Ethology 110:245–255. https://doi.org/10.1111/j.1439-0310.2004.00965.x
Dreher CE, Pröhl H (2014) Multiple sexual signals: calls over colors for mate attraction in an aposematic, color-diverse poison frog. Front Ecol Evol 2:22. https://doi.org/10.3389/fevo.2014.00022
Dugas MB (2018) Simple observations with complex implications: what we have learned and can learn about parental care from a frog that feeds its young. Zool Anz 273:192–202. https://doi.org/10.1016/j.jcz.2017.11.012
Dugas MB, Richards-Zawacki CL (2015) A captive breeding experiment reveals no evidence of reproductive isolation among lineages of a polytypic poison frog. Biol J Linn Soc 116:52–62. https://doi.org/10.1111/bij.12571
Edward DA, Chapman T (2011) The evolution and significance of male mate choice. Trends Ecol Evol 26:647–654. https://doi.org/10.1016/j.tree.2011.07.012
Gade M (2015) Female mate choice in a mainland population of the strawberry poison frog Oophaga pumilio. John Carroll University
Gade MR, Hill M, Saporito RA (2016) Color assortative mating in a mainland population of the poison frog Oophaga pumilio. Ethology 122:851–858. https://doi.org/10.1111/eth.12533
Gerhardt HC, Tanner SD, Corrigan CM, Walton HC (2000) Female preference functions based on call duration in the gray tree frog (Hyla versicolor). Behav Ecol 11:663–669. https://doi.org/10.1093/beheco/11.6.663
Gwynne DT (1991) Sexual competition among females: What causes courtship-role reversal? Trends Ecol Evol 6:118–121. https://doi.org/10.1016/0169-5347(91)90089-G
Haase A, Pröhl H (2002) Female activity patterns and aggressiveness in the strawberry poison frog Dendrobates pumilio (Anura: Dendrobatidae). Amphibia-Reptilia 23:129–140. https://doi.org/10.1163/156853802760061778
Janicke T, Häderer IK, Lajeunesse MJ, Anthes N (2016) Darwinian sex roles confirmed across the animal kingdom. Sci Adv 2:e1500983. https://doi.org/10.1126/sciadv.1500983
Janicke T, Ritchie MG, Morrow EH, Marie-Orleach L (2018) Sexual selection predicts species richness across the animal kingdom. Proc R Soc B 285:20180173. https://doi.org/10.1098/rspb.2018.0173
Johnstone RA, Reynolds JD, Deutsch JC (1996) Mutual mate choice and sex differences in choosiness. Evolution 50:1382–1391. https://doi.org/10.1111/j.1558-5646.1996.tb03912.x
Maan ME, Cummings ME (2008) Female preferences for aposematic signal components in a polymorphic poison frog. Evolution 62:2334–2345. https://doi.org/10.1111/j.1558-5646.2008.00454.x
Maan ME, Cummings ME (2009) Sexual dimorphism and directional sexual selection on aposematic signals in a poison frog. P Natl Acad Sci USA 106:19072–19077. https://doi.org/10.1073/pnas.0903327106
McVey ME, Zahary RG, Perry D, MacDougal J (1981) Territoriality and homing behavior in the poison dart frog (Dendrobates pumilio). Copeia 1981:1–8. https://doi.org/10.2307/1444035
Meuche I, Linsenmair KE, Pröhl H (2011) Female territoriality in the strawberry poison frog (Oophaga pumilio). Copeia 2011:351–356. https://doi.org/10.1643/CE-08-135
Nakahashi W (2008) Quantitative genetic models of sexual selection by male choice. Theor Popul Biol 74:167–181. https://doi.org/10.1016/j.tpb.2008.06.001
Palacios-Rodríguez P, González-Santoro M, Amézquita A, Brunetti AE (2022) Sexual dichromatism in a cryptic poison frog is correlated with female tadpole transport. Evol Ecol 36:153–162. https://doi.org/10.1007/s10682-021-10147-4
Peignier M, Bégué L, Gieseke A, Petri D, Ringler M, Ringler E (2022) Mate choice in a promiscuous poison frog. Ethology 128:693–703. https://doi.org/10.1111/eth.13331
Pröhl H (2002) Population differences in female resource abundance, adult sex ratio, and male mating success in Dendrobates pumilio. Behav Ecol 13:175–181. https://doi.org/10.1093/beheco/13.2.175
Pröhl H, Berke O (2001) Spatial distributions of male and female strawberry poison frogs and their relation to female reproductive resources. Oecologia 129:534–542. https://doi.org/10.1007/s004420100751
Pröhl H, Hödl W (1999) Parental investment, potential reproductive rates, and mating system in the strawberry dart-poison frog, Dendrobates pumilio. Behav Ecol Sociobiol 46:215–220. https://doi.org/10.1007/s002650050612
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna Austria https://www.R-project.org/
Reynolds RG, Fitzpatrick BM (2007) Assortative mating in poison-dart frogs based on an ecologically important trait. Evolution 61:2253–2259. https://doi.org/10.1111/j.1558-5646.2007.00174.x
Rice WR, Gaines SD (1994) ‘Heads I win, tails you lose’: testing directional alternative hypotheses in ecological and evolutionary research. Trends Ecol Evol 9:235–237. https://doi.org/10.1016/0169-5347(94)90258-5
Richards-Zawacki CL, Cummings ME (2011) Intraspecific reproductive character displacement in a polymorphic poison dart frog, Dendrobates pumilio. Evolution 65:259–267. https://doi.org/10.1111/j.1558-5646.2010.01124.x
Ron SR (2008) The evolution of female mate choice for complex calls in túngara frogs. Anim Behav 76:1783–1794. https://doi.org/10.1016/j.anbehav.2008.07.024
RStudio Team (2019) RStudio: Integrated Development for R. RStudio Inc., Boston MA http://www.rstudio.com/
Sandvik M, Rosenqvist G, Berglund A (2000) Male and female mate choice affects offspring quality in a sex–role–reversed pipefish. Proc R Soc Lond B 267:2151–2155. https://doi.org/10.1098/rspb.2000.1262
Shahandeh MP, Pischedda A, Turner TL (2018) Male mate choice via cuticular hydrocarbon pheromones drives reproductive isolation between Drosophila species. Evolution 72:123–135. https://doi.org/10.1111/evo.13389
Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K (2004) Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J Exp Biol 207:2471–2485. https://doi.org/10.1242/jeb.01047
Summers K (1989) Sexual selection and intra-female competition in the green poison-dart frog, Dendrobates auratus. Anim Behav 37:797–805. https://doi.org/10.1016/0003-3472(89)90064-X
Summers K (1990) Paternal care and the cost of polygyny in the green dart-poison frog. Behav Ecol Sociobiol 27:307–313
Summers K, Cronin TW, Kennedy T (2003) Variation in spectral reflectance among populations of Dendrobates pumilio, the strawberry poison frog, in the Bocas del Toro Archipelago, Panama. J Biogeogr 30:35–53. https://doi.org/10.1046/j.1365-2699.2003.00795.x
Summers K, Symula R, Clough M, Cronin T (1999) Visual mate choice in poison frogs. Proc R Soc Lond B 266:2141–2145. https://doi.org/10.1098/rspb.1999.0900
Swierk L, Langkilde T (2021) Size-assortative mating in explosive breeders: a case study of adaptive male mate choice in anurans. Behaviour 158:849–868. https://doi.org/10.1163/1568539X-bja10098
ten Cate C, Vos DR (1999) Sexual imprinting and evolutionary processes in birds: a reassessment. Adv Stud Behav 28:1–31. https://doi.org/10.1016/S0065-3454(08)60214-4
Tudor MS, Morris MR (2008) Variation in male mate preference for female size in the swordtail Xiphophorus malinche. Behaviour 146:727–740. https://doi.org/10.1163/156853909X446172
Tuschhoff E, Wiens JJ (2023) Evolution of sexually selected traits across animals. Front Ecol Evol 11:1042747. https://doi.org/10.3389/fevo.2023.1042747
Ursprung E, Ringler M, Jehle R, Hödl W (2011) Strong male/male competition allows for nonchoosy females: high levels of polygynandry in a territorial frog with paternal care. Mol Ecol 20:1759–1771. https://doi.org/10.1111/j.1365-294X.2011.05056.x
Vos DR (1995) The role of sexual imprinting for sex recognition in zebra finches: a difference between males and females. Anim Behav 50:645–653. https://doi.org/10.1016/0003-3472(95)80126-X
Yang Y, Blomenkamp S, Dugas MB, Richards-Zawacki CL, Pröhl H (2019a) Mate choice versus mate preference: Inferences about color-assortative mating differ between field and lab assays of poison frog behavior. Am Nat 193:598–607. https://doi.org/10.1086/702249
Yang Y, Dugas MB, Sudekum HJ, Murphy SN, Richards-Zawacki CL (2018) Male–male aggression is unlikely to stabilize a poison frog polymorphism. J Evol Biol 31:457–468. https://doi.org/10.1111/jeb.13243
Yang Y, Richards-Zawacki CL, Devar A, Dugas MB (2016) Poison frog color morphs express assortative mate preferences in allopatry but not sympatry. Evolution 70:2778–2788. https://doi.org/10.1111/evo.13079
Yang Y, Servedio MR, Richards-Zawacki CL (2019b) Imprinting sets the stage for speciation. Nature 574:99–102. https://doi.org/10.1038/s41586-019-1599-z
Acknowledgements
We thank the reviewers and editors for valuable comments that improved our manuscript. Thanks also to Miranda Kosowsky and Caitlin Nordheim for assistance with animal care and husbandry, as well as Yusan Yang for developing the behavioral assay protocol that we adapted for use in our study. We also thank the Departmental Honors Committee at the University of Pittsburgh’s Department of Biological Sciences for their assistance with guiding and reviewing this research.
Funding
This research was supported by a grant from the National Science Foundation (2050358) and start-up funds from the University of Pittsburgh to CLR-Z.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The use of animals in this study adheres to the guidelines set forth by the Animal Behavior Society/Association for the Study of Animal Behaviour. All procedures that involve animals were reviewed and approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee (IACUC, protocol #21089903). All authors confirm that the welfare of animals was prioritized and respected in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by E. Ringler
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lehman, M.R., González-Santoro, M. & Richards-Zawacki, C.L. Little evidence for color- or size-based mating preferences by male strawberry poison frogs (Oophaga pumilio). Behav Ecol Sociobiol 78, 19 (2024). https://doi.org/10.1007/s00265-024-03436-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03436-9