Abstract
Behaviour is considered among the most important factors in colonising new habitats. While population divergence in behaviour is well-documented, intraspecific variation in exploratory behaviour in species with populations successfully colonising and adapting to extreme (compared to the ‘typical’) habitats is less understood. Here, by studying surface- vs. cave-adapted populations of water louse (Asellus aquaticus), we tested whether (i) adaptation to the special, ecologically isolated cave habitat includes a decrease in explorativeness and (ii) recent, surface-type cave colonists are more explorative than their surface conspecifics from the source population. We repeatedly tested dispersal related novel area exploration and dispersal speed in both the presence and absence of light. We found that surface populations showed higher behavioural activity in dark than in light, and they were more explorative and dispersed faster than their cave conspecifics. Recent colonists showed a trend of higher dispersal speed compared to their source surface population. We suggest that extreme and isolated habitats like caves might work as ‘dispersal traps’ following successful colonisation, because adaptation to these habitats includes the reduction of explorativeness. Furthermore, we suggest that individuals with higher explorativeness are likely to colonise markedly new environments. Finally, we provide experimental evidence about surface A. aquaticus moving more in dark than in light.
Significance statement
Environmental conditions in caves are differing drastically from those of the surface. Consequently, animals colonising subterranean habitats are subject to different selective forces than those experienced by the ancestral surface-living population. Behaviour is believed to be a key factor in successful colonisation to novel habitats; however, intraspecific behavioural variation in species with both surface- and cave-adapted populations is less known. Here, we compared dispersal related novel area exploration and dispersal speed across surface and cave-adapted populations of the freshwater crustacean Asellus aquaticus. Our results show that cave-adapted A. aquaticus are significantly less explorative and disperse slower than surface-type populations, indicating that caves may act as ‘dispersal traps’, where adaptation includes the loss of explorativeness. Also, recent cave colonists show a trend to be faster dispersers than peers from the surface source population, suggesting that individuals with higher explorativeness are likely to colonise markedly different new environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heritable variation in behavioural traits within species and populations is an important factor for successful colonisation of novel habitats or withstanding environmental variation (Morse 1980). Consistent between-individual variation in behaviour over time and across ecological situations within population (i.e. animal personality) is a common and widespread phenomenon across the animal kingdom (for reviews and meta-analyses see e.g. Sih et al. 2004; Réale et al. 2007; Bell et al. 2009; Dingemanse and Wolf 2010; Garamszegi et al. 2013). Furthermore, a growing number of theoretical and empirical works suggest that individuals settle in habitats, which suits their personality (here: individual behavioural configuration) the best, implying the existence of personality matching habitat choice (Edelaar et al. 2008; Jacob et al. 2015; Saltz et al. 2018; Saltz 2019). Between-individual variability in dispersal is associated with differences in various behavioural traits: dispersers (or colonisers) seem to be more risk-taking, more explorative, asocial or aggressive than non-dispersers (Blumstein et al. 2009; Cote et al. 2010a, b; Spiegel et al. 2017). Between-individual behavioural variation may be especially important in the colonisation of markedly different new habitats. By ‘markedly different habitat’, we mean a new habitat that has fundamentally distinct environmental properties compared to the habitats occupied by the given species in general. In other words, such habitat differs in one or more major environmental parameters (e.g. a marked shift in mean temperature or composition of food sources, presence of significant new predator/competitor species) from that of the colonising population’s habitat. Such marked habitat shifts are not frequent for the majority of species, but a good example can be the colonisation of caves by surface species.
Caves and related subterranean habitats are characterised by the absence of light, food scarcity and simplified communities, and are strongly buffered against daily, seasonal and yearly environmental variations (Romero 2009; Borowsky and Cohen 2013; Culver and Pipan 2019). Adaptation to the cave environment, often summarised as troglomorphism (e.g. eye degeneration, depigmentation, lowered metabolism, extended life cycles, absence of circadian rhythm; see Porter and Crandall 2003; Mejía-Ortíz et al. 2006), makes it almost impossible for a troglomorphic population to leave the cave and successfully (re)colonise surface habitats. In accordance with this notion, recent empirical studies indeed suggest that dispersal ability of troglobionts (obligate cave dwellers) is, if not zero, extremely limited (see Lefébure et al. 2007; Trontelj et al. 2009; Stern et al. 2017; Balogh et al. 2020). Hence, markedly different and isolated habitats like caves might work as a ‘dispersal trap’ after colonisation by surface populations. Two main hypotheses are explaining the transition from surface to subterranean habitats. First, the ‘adaptive shift’ hypothesis suggests that populations of a surface (epigean) species invade subterranean (hypogean) habitats to exploit novel resources (Howarth 1980). Second, the ‘climatic relict’ hypothesis suggests that a species may be forced to colonise underground habitats to avoid uninhabitable environmental conditions on the surface, for instance, cave colonisation can be triggered by glaciation events (Peck and Finston 1993; Rivera et al. 2002; Danielopol and Rouch 2005; Juan and Emerson 2010). However, not only climate change can induce cave colonisations, but also other environmental challenges, like predation pressure, can cause the same effect (Romero 1985; Tobler 2009).
At any rate, the evolution of dispersal-related behaviours following the successful colonisation of markedly different and isolated habitats acting as dispersal traps is rarely studied. Generally, cave-adapted organisms are expected to have lowered metabolic rates and decreased movement activity (see Hüppop 2000; Hervant et al. 2001), an extreme example being the olm (Proteus anguinus), which shows a surprisingly low movement activity, revealed by a recent capture-mark-recapture study (Balázs et al. 2020). Theory also suggest that environments with high temporal stability and predictability should favour reduced exploration (Sih et al. 2004; Careau et al. 2009). In line with this, Mettke-Hofmann et al. (2002) found that parrot species that live in complex habitats, such as forest edges, are more explorative than species found in simpler habitats, implying that habitat complexity favours high explorativeness. Hence, dispersal-related exploratory behaviour is expected to show a clear decrease in cave-adapted organisms; however, empirical data are scarce at best, and results seem to be inconclusive in this regard. For example, the only laboratory experiment to our knowledge that directly studied dispersal-related novel area exploration in surface and cave-adapted populations of the freshwater crustacean Asellus aquaticus found no difference between the morphotypes (Brengdahl 2016). Furthermore, we do not know whether personality variation within the source population plays a role in the colonisation, i.e. whether the boldest, most explorative individuals enter the markedly different new habitat, or whether colonisation happens randomly due to environmental pressures explained by either or both of the adaptive shift and climatic relict hypotheses.
Here, we aimed to study the link between exploratory behaviour and cave colonisation on different levels. First, we were interested in how exploratory behaviour varies between surface and cave populations. Since we assumed that caves act as dispersal traps and the environment within caves has low variability, we predicted that explorativeness shows a significant decrease in cave populations compared to surface ones. Second, we tested whether individuals actually dispersing into caves are different from the source population’s mean in their explorativeness. We predicted that the most explorative individuals from a population are the ones entering the markedly different new habitat, i.e. potential colonists express higher explorativeness than the average in their source population. Our model was the water louse, Asellus aquaticus (Linnaeus 1758). The species is a widespread freshwater crustacean that colonised some caves in Europe, where its populations exhibit ‘troglomorphic’ phenotypes (lack of pigmentation, loss of vision, enlarged sensory, ambulatory appendages, etc.) (Prevorčnik et al. 2004; Konec et al. 2015).
Material and methods
Study system and sampling
We studied three surface populations and one cave population. Molnár János Cave is a hypogene (water in the cave is not coming from the surface) cave filled with thermal water of constant temperature of 23–24 °C (Erőss et al. 2006; Bodor et al. 2015). There is no exogenous food in the cave, only endogenous bacteria-forming mats (see Herczeg et al. 2020 for details). The cave community is extremely simple, and there are no predators of A. aquaticus present. The cave is connected to a surface pond formed by the water outflow right at the cave entrance (47.518277° N, 19.035999° E), called Malom Lake, and there is no physical barrier between the habitats. Despite this fact, the troglomorphic population in Molnár János Cave is genetically isolated from the surrounding surface populations (including Malom Lake) for at least 60 000 years (Pérez-Moreno et al. 2017). The water temperature in Malom Lake is identical to that of the cave and constant all year round. However, Malom Lake is subjected to the natural surface light regime and can be seen otherwise as a typical surface habitat, including the presence of fish predators, i.e. guppies (Poecilia reticulata) that were introduced here during the twentieth century (Berczik 1956) and occur in extremely high density as native fish are absent.
We sampled Molnár János Cave, Malom Lake, recent cave colonists (surface type individuals coming from Malom Lake, collected in Molnár János Cave ca. 250 m from the cave entrance), and two surface populations from the vicinity, Gőtés Lake (47.354357° N, 19.22980° E) and the Dunakeszi Peat-moor (47.615613° N, 19.126392° E) between 16 and 18 May 2019. Samples were collected by hand sorting with a mesh net, except for the animals from the Molnár János Cave, where a modified Sket bottle was used (Chevaldonné et al. 2008) and cave diving was necessary. We considered collected individuals larger than 4 mm as adults (Hasu et al. 2007; Bloor 2011). As gravid females of A. aquaticus form a brood pouch (marsupium) to carry offspring (see Lafuente et al. 2021) and likely display different behavioural activity, we only used non-gravid females to avoid any bias in this respect. Gőtés Lake and Dunakeszi Peat-moor populations experience natural surface light regime and temperature fluctuations of normal, non-thermal freshwaters typical to the region. These populations represent typical surface habitats widespread in the region, and we chose them randomly. Surface type individuals recently entering the cave (hereafter ‘potential colonists’) from Malom Lake most likely do not form a population, but for simplicity’s sake, we will refer to all compared groups (four populations and the colonists) as ‘populations’.
All animals were transported immediately after capture to the aquacultural facilities of the Eötvös Loránd University (Budapest, Hungary) and housed individually in 90 × 25 mm (diameter and height, respectively) plastic Petri dishes, with sandpaper-coarsened bottoms aiding the animals’ normal movement (Fišer et al. 2019). Cave and colonist individuals were kept in constant darkness, while surface populations in a 16-h light:8-h dark daily light cycle (for the methodology, see the next section). The temperature in the lab was set to 23–24 °C, which is the constant water temperature in the Molnár János Cave and Malom Lake, and within the natural range of the other surface populations at this time of sampling. Water collected from the natural habitats was used for keeping and testing throughout the laboratory period. We had the following number of adult individuals in the tests reported here: Molnár János Cave: 10 males (M)/8 females (F), colonists: 8 M/5 F, Malom Lake: 17 M/13 F, Gőtés Lake: 14 M/13 F; Dunakeszi Peat-moor: 14 M/15 F.
Behavioural assays
We used custom-built chambers for both keeping the study animals under the above-detailed light settings and video-recording their behaviour. The chambers had the following dimensions: 100 cm length × 55 cm width × 105 cm height. The chambers’ sides and top were covered by black plastic sheets to block any incoming light. The chambers were equipped with two light sources. On the top, we installed LEDs imitating daylight (colour temperature = 4500 K, colour rendering index > 90), while on the bottom, we installed infrared LEDs (wavelength = 920 nm). This infrared wavelength is outside of the visible range of A. aquaticus (Dember and Richman 2012). The lights could be switched on/off from the outside. Daylights were used to produce the planned daily light regime, while infrared LEDs were used to make the video-records in either light or dark. On the bottom of the chambers, we placed an opaque plexiglass sheet to diffuse the infrared light evenly and to provide a platform for the Petri dishes. In all chambers, we mounted four webcams (Logitech C920 Full HD; Logitech, Lausanne, Switzerland) to the top that were modified for infrared video-recording. We used the OBS Studio software (OBS Studio Contributors) to capture videos (5 frames per second, HD [1280 × 720 pixels] resolution).
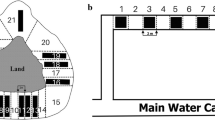
The assays took place between 21 May and 1 June on 12 consecutive days. Animals were allowed to acclimate under their natural light regime without any disturbance. Potential colonists were acclimated in dark, since they were collected from the cave. During acclimation and testing, we provided no food for the test individuals. The tests started approximately at 11.00 am (UTC + 2.00) on each day. Individual behaviour was tested in chambers described above. To test novel area exploration and dispersal speed, we used a cluster of 18 open-top mazes made of Plexiglas (see Fig. 1). The lateral walls were black, while the bottom was transparent and sandpaper-coarsened to enable animals’ normal movement (Fišer et al. 2019). A long division wall was installed in the middle of each maze (5.4 cm × 44.25 cm × 3 cm, width × length × height, respectively) that resulted in a U-shaped compartment. In each branch of the maze, we put seven removable obstacles (2 cm wide). This, considering the tip of the division wall too, resulted in 15 obstacles an animal had to pass around to get from one to the other end of the maze. Each population was randomly split into two groups: One of them was tested in the presence of light, while the other group in darkness. After 48 h, treatments were changed; thus, each subgroup was measured in light and dark alternately. Altogether, every individual was tested six times (three times in light + three times in dark), resulting in a total of 702 observations. We have to note here that the light treatment is completely unnatural for the cave population and can only be used to test for light avoidance (based on genetic-studies, cave-dwelling A. aquaticus probably retained the ability to detect light; Pérez-Moreno et al. 2018). However, both treatments are biologically relevant for the surface populations. It is noteworthy that even though the species is intuitively treated as diurnal, there is a field study indicating that A. aquaticus might move more during the night than during the day (Andrikovics 1981). We could test a maximum of three groups daily and testing order was randomised each day. Arenas were filled with water from the habitat of the tested population. We used a pipette to transport individuals from their Petri dish to a transparent cylinder placed at the starting point of each maze. The maze used for each individual was randomly selected beforehand. Once all individuals were placed in the maze, we waited 5 min and started recording (lasted for 60 min) and removed the cylinders to allow free movement.
Schematic representation of one experimental arena with 18 mazes (top). Thick lines indicate a single maze of the arena with an individual in the starting position (bottom); note that the animal on the picture was magnified for better visibility. Asellus aquaticus were individually tested for crossing the obstacles and exploring the maze; total number of obstacles crossed in any direction represented ‘novel area exploration’ (an animal was considered to cross an obstacle when its whole body crossed) and ‘dispersal speed’ (for individuals that reached the farthest possible obstacle [15], the sequential number of this was divided by the actual time [s] required to reach it, while in cases when individuals did not reach the farthest possible obstacle, we divided the sequential number of the farthest obstacle crossed by 3600 s, i.e. the entire duration of the experiment)
We extracted two dispersal-related variables from the videos. Total number of obstacles crossed in any directions (an animal was considered to cross an obstacle when its whole body crossed) correlated highly with the farthest obstacle crossed (Spearman’s rho = 0.87; 95% confidence interval (CI) = 0.84–0.89), and we chose the former to describe ‘novel area exploration’ because it involves an element of thoroughness. The second variable was ‘dispersal speed’ (for individuals that reached the farthest possible obstacle [15], the sequential number of this was divided by the time [s] required to reach it, while in cases when individuals did not reach the farthest possible obstacle, we divided the sequential number of the farthest obstacle crossed by 3600 s, i.e. the entire duration of the experiment). We emphasise that both variables are describing exploration sensu Réale et al. (2007), i.e. both variables are describing movement behaviour in a new (potentially risky) situation; hence, they are clearly different from activity sensu Réale et al. (2007), which must be measured in a non-novel and non-risky situation.
Statistical analyses
To analyse population, treatment and sex effects on novel area exploration, a generalised linear mixed model (GLMM) with negative binomial distribution and logit link function was used. We chose this method, as transformation (log or square root) of count data is generally contraindicated (O’Hara and Kotze 2010). We added ‘population’ (the four sampled populations and the potential colonists), ‘treatment’ (light vs. dark), sex and their interactions as fixed effects. Given that the interpretation of three-way factorial interactions can be problematic, and to avoid overparameterisation, we included only the two-way interactions. To control for habituation to the test setup, we added the standardised (mean = 0, sd = 1) order of trials (hereafter, ‘time’) as a single fixed effect. We added individual identity as a random intercept. We also added random slopes (individual × time) as a random term, but left it in the final model only if it improved model fit. Error distribution and link function applied in the GLMMs were chosen after inspection of Q-Q plots of the model residuals. Fixed effects were tested by Wald’s chi-squared tests and random effects by likelihood ratio tests. P values for the likelihood ratio tests were calculated following Zuur et al. (2009). We extracted the model’s estimated marginal means using the emmeans package (Lenth 2019). To compare groups, we looked for the presence/absence of overlaps between 85% CIs following Payton et al. (2003), who demonstrated that the lack of overlap in 83–84% CIs is analogous to a P-value < 0.05. We report the proportion of explained variance by the fixed factors (marginal R2) and by both fixed and random factors (conditional R2) available in the MuMIn package (Barton 2009) based on the method of Nakagawa and Schielzeth (2013). Dispersal speed data were square root transformed to achieve normal distribution of the model residuals. We analysed this behavioural variable using a linear mixed model (LMM) built in the same way as described for the GLMM above. We built both the GLMM and the LMM with the R packages lme4 and lmerTest (Bates et al. 2015; Kuznetsova et al. 2016, respectively) in R 4.1.0 (R Developmental Core Team 2021).
The rptR add-on package (Stoffel et al. 2017) was used to calculate repeatability (a statistical test for the presence and ‘strength’ of animal personality, i.e. consistent among-individual differences over time or across ecological contexts; see Réale et al. 2007; Sih et al. 2012; Niemelä and Dingemanse 2018). Enhanced agreement repeatability (hereafter: eaR) for novel area exploration and dispersal speed in the pooled sample (i.e. all populations combined) was calculated separately for light regimes. This method allows us to fit improved models, in which the variance explained by fixed effects is calculated by the variance in the linear predictor, including the fixed effects’ variance in the denominator (see Stoffel et al. 2017). Models were parameterised as described above; nevertheless, as we fitted them separately for different light regimes, treatment effect and its interactions were omitted. We built GLMMs for the negative binomially distributed novel area exploration data, following the methods of Nakagawa and Schielzeth (2010), which utilise a multiplicative overdispersion GLMM with a logit-link and using penalised quasi-likelihood (PQL) estimation for repeatability on the original scale. Significance of eaR estimates (i.e. for random terms) was provided by randomisation tests, giving robust measures of statistical significance in the case of non-Gaussian data (Nakagawa and Schielzeth 2010). However, we report repeatabilities estimated on the underlying latent (link) scale as most original-scale repeatabilities are conditional for non-Gaussian data (Nakagawa and Schielzeth 2010). Quantification of uncertainty for the variance explained by fixed effects (as for other variance components) was provided by parametric bootstrapping. LMMs were run to estimate repeatability for dispersal speed. CIs were calculated by nonparametric bootstrapping, while significance for eaR estimates is provided by likelihood ratio test, both sampled at each 1000th iteration.
Results
Repeatability estimates for novel area exploration and dispersal speed are given in Table S1. For novel area exploration, estimates in the pooled sample indicated significant, moderate (compared to Bell et al. 2009) repeatability in both treatments (light: eaR = 0.29 (95% CI = 0.13–0.35); dark: 0.22 (0.09–0.28)). Repeatability for dispersal speed in the pooled sample was present only in the presence of light, showing moderate strength (eaR = 0.21 (0.11–0.33)).
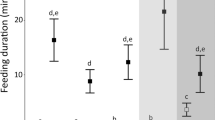
Population, treatment and their interaction all affected novel area exploration (Table 1). All other populations in both treatments showed higher novel area exploration than cave individuals in dark (Fig. 2). The cave population showed increased novel area exploration comparable to those of the surface populations in the — for them unnatural — light treatment. Surface populations explored more in dark than in light, the trend being weak in Dunakeszi Peat-moor. Treatment had no effect on the potential colonists’ exploration. Colonists had higher exploration in light, than surface populations, including their source population (Malom Lake), but their exploration in dark was similar to the surface populations. Furthermore, we found a significant sex × treatment interaction effect (Fig. 3): Males were more active than females in dark, but not in light (see Table S3). Habituation was also significant: individuals became less active by time (Fig S1a). Finally, individual differences in novel area exploration and habituation were both significant. The fixed effects explained 36.9% of the total variance, and the full models explained 74.2%.
Novel area exploration in the absence vs. presence of light in the four tested populations of Asellus aquaticus (significant population × environment interaction). White background indicates surface type individuals, while light grey indicates surface type individuals from the Malom Lake found in the cave, and dark grey indicates cave-adapted individuals. DM, Dunakeszi Peat-moor; GL, Gőtés Lake; ML, Malom Lake; C(ML), Colonists from the Malom Lake; MJC, Molnár János Cave. Backtransformed estimated marginal means and 85% confidence interval (CI) are shown. Note that lack of overlap in 83–84% CIs is analogous to a P value < 0.05 (Payton et al. 2003)
Novel area exploration in the absence vs. presence of light in the female and male Asellus aquaticus (significant sex × environment interaction). Backtransformed estimated marginal means and 85% confidence interval (CI) are shown. Note that lack of overlap in 83–84% CIs is analogous to a P value < 0.05 (Payton et al. 2003)
We found significant population and population × environment interaction effects on dispersal speed (Table 1). All populations in dark and Dunakeszi Peat-moor and potential colonists in light dispersed faster than the cave population in dark (Fig. 4). Cave A. aquaticus showed comparable dispersal speed to the surface populations in the — for them unnatural — light treatment. Potential colonists from the Malom Lake dispersed faster than their source population (Malom Lake) in light and tended to do so in in dark (see Fig. 4). We also found significant habituation in dispersal speed, with a decrease by time (Fig S1b). Individual differences in dispersal speed were significant. The fixed effects explained 12.2% of the total variance, and the full models explained 27.2%.
Dispersal speed in the absence vs. presence of light in the four tested populations of Asellus aquaticus (significant population × environment interaction). White background indicates surface type individuals, while light grey indicates surface type individuals from the Malom Lake found in the cave, and dark grey indicates cave-adapted individuals. DM, Dunakeszi Peat-moor; GL, Gőtés Lake; ML, Malom Lake; C(ML), Colonists from the Malom Lake; MJC, Molnár János Cave. Estimated marginal means and 85% confidence interval (CI) are shown. Note that lack of overlap in 83–84% CIs is analogous to a P value < 0.05 (Payton et al. 2003)
Discussion
Population divergence in behavioural types
Our understanding of the evolution of dispersal-related behaviours in populations adapted to isolated and specialised habitats, i.e. dispersal traps, is rather incomplete. We expected generalist, surface populations of A. aquaticus to be more explorative (i.e. showing higher novel area exploration and being faster dispersers) than conspecifics adapted to the Molnár János Cave. This expectation is supported by the results. All surface populations showed higher exploration, irrespective of light treatment, than cave-adapted individuals in the dark (the natural condition for cave individuals). The dispersal speed patterns were similar, when surface populations were tested in the dark; however, the divergence was weak in Gőtés Lake and Malom Lake individuals tested in light. A. aquaticus in the Molnár János cave are adapted to an isolated, stable, predictable and (compared to the surface habitats) homogenous environment with no predators and high quantity of probably low-quality endogenous food (see Herczeg et al. 2020; 2022). Thus, this special environment most probably acts as a dispersal trap, i.e. the presumably photophobic cave-adapted individuals are unlikely to leave the cave. Increased behavioural activity, comparable to surface populations, could be observed in the Molnár János Cave population in the presence of light. In environments with adverse stimuli, it is expected that novel area exploration is increased as it enhances the chance of finding a more suitable environmental patch, while slowing down can indicate the onset of favourable conditions (Fraenkel and Gunn 1961; Breed and Moore 2021). In our case, increased activity is likely caused by photophobia in the cave-adapted individuals (Janzer and Ludwig 1952; Fišer et al. 2016). Note that despite the marked eye reduction of the cave-adapted individuals, they still preserved the ability to detect light (Pérez-Moreno et al. 2018).
Because of the lack of large predators in most subterranean habitats, behaviour of cave-adapted individuals is virtually not affected by trade-offs between foraging profitability and predation risk. Furthermore, food availability in the majority of caves is limited compared to surface habitats (Culver and Pipan 2019). Therefore, increased activity in caves is expected to find food fulfilling energetic demands (Culver and Poulson 1971; Hüppop 2000). On the other hand, as increased movement activity itself leads to higher energy expenditure, prolonged food shortage was shown to reduce locomotor activity (together with reduced metabolic and respiratory rates) in several hypogean taxa (e.g. Niphargus sp. and Stenasellus virei Hervant et al. 1997; Hervant and Renault 2002; Proteus anguinus Hervant et al. 2001). Although endogenous bacterial mats in the Molnár János Cave can be found in large amounts, our recent findings indicate that cave-adapted A. aquaticus in the Molnár János Cave not just maintained the ability to feed on decaying leaf litter, but actually, this food is preferred over bacterial mats despite the latter are the only available source of food in the cave (Herczeg et al. 2020, 2022). Regarding the nutritional content of Molnár János Cave bacterial mats, we possess no exact information, but it was shown recently that potentially toxic metals (e.g. As, Hg, Pb, Sn, Sr, Zn) may accumulate in the biofilm (see Dobosy et al. 2016; Enyedi et al. 2019). This might result in poor quality diet and, along with our previous behavioural observations (Herczeg et al. 2020, 2022), indirectly indicates that bacterial mats in this cave might be seen as an obstacle for colonisation (surface populations unconnected to the cave avoided bacteria almost entirely) and that the Molnár János Cave population might be somewhat food deprived after all. In a previous study (Berisha et al. 2022), we found elevated movement activity in the Molnár János cave population compared to surface populations in a familiar environment, while in the present study, we found decreased explorativeness in the cave population in a novel environment. Based on these results, we suggest that cave-adapted individuals moves more in a familiar, perceived risk-free situation, probably as an adaptation to the lack of predators to maximise foraging success, but they are less active in a novel situation.
Light-induced behavioural plasticity and sexual dimorphism
In addition to the detected photophobia in cave-adapted A. aquaticus (see above), we were curious about effect of light regime on the behaviour of surface populations too. While these populations are intuitively expected to be diurnal, and in fact, they are active during the day, Andrikovics (1981) showed that surface-dwelling A. aquaticus are three times more likely to be found in funnel traps during the night than during the day. This suggests an increased night-time activity of A. aquaticus, plausibly as an outcome of low predation rate in darkness. Our results are supporting Andrikovics’s (1981) observation: surface populations showed increased novel area exploration in dark compared to light, showing similar trends in dispersal speed. Based on these results, we have to reject the notion of the primarily diurnal activity of the species. Increased behavioural activity in darkness could also explain why A. aquaticus is successful in colonising subterranean habitats.
We also found male A. aquaticus to be more explorative than females in darkness. Male A. aquaticus were previously shown to seek actively for mates during the mating season, unlike females (Bertin et al. 2002), and males are performing precopula or mate guarding (Thompson and Manning 1981), which in the significant majority of cases followed by fertilisation (Eroukhmanoff et al. 2009). Therefore, the higher behavioural activity of males observed in our study can be explained by reproductive behaviour. Male-biased sexual dimorphism in exploration, expressed only in dark, further emphasises the importance of night activity for the species. However, this question definitely needs further studies.
Behaviour of potential cave colonists
Personality related dispersal is a well-documented phenomenon (e.g. Blumstein et al. 2009; Cote et al. 2010a; Spiegel et al. 2017). However, less is known about personality related colonisation of markedly different habitats (but see studies about colonising urban environments, e.g. Schuett et al. 2018; Baxter-Gilbert et al. 2019). Cave colonisation by surface populations is explained by two hypotheses: the first assumes that the driving forces are the new, yet unexploited resources provided by the caves, while the second states that caves serve as refuges from the harsh surface environments (Howarth 1980; Peck and Finston 1993; Rivera et al 2002; Danielopol and Rouch 2005; Juan and Emerson 2010). Irrespective of the environmental driver, a subset of surface individuals must enter the new cave environment for the start of the colonisation process. Even if we consider the high behavioural activity of A. aquaticus in total darkness (see above) as a sign of exaptation to the cave life (see Pérez-Moreno et al. 2017), aquatic cave environments are still different (simple and stable biotic and abiotic environment) from surface water bodies. This is especially true for the Molnár János Cave, where the only food sources are endogenous bacterial mats absent from the surface habitats. These bacterial mats are unpreferred compared to surface food or unnatural, but nutritious food even by the cave population (see Herczeg et al. 2020; 2022). Theory predicts that individual dispersal within the same habitat type is linked to personality; as intuitively expected, bolder (i.e. more active, explorative, risk-taking) individuals disperse further than their shier conspecifics (Dingemanse et al. 2003; Cote et al. 2010a, b, 2017; Myles-Gonzalez et al. 2015). Colonisation of new habitats (in an urbanisation context) was also linked to personality in a similar way (Atwell et al. 2012; Bókony et al. 2012; Schuett et al. 2018; Baxter-Gilbert et al. 2019). Based on this, we predicted that surface A. aquaticus individuals from the Malom Lake that were found in the cave (‘potential colonists’) are more explorative than the average Malom Lake individual. Note that ‘accidental’ entrance to the cave is highly unlikely, as the Boltív Spring is flowing from the Molnár János Cave to the Malom Lake, which means that potential colonists have to move actively against the current to enter the cave. We have to also note that we have no information on how much time potential colonists have spent in the cave; it is equally plausible that they entered recently (i.e. within days) or that their parents entered the cave and they were born there.
Regarding novel area exploration, potential colonists showed higher explorativeness under both light treatments, than the other surface populations (including Malom Lake) in light, while they did not differ in dark. The difference was similar for dispersal speed in light, but colonists also tended to disperse faster in dark. Hence, it seems plausible to suggest that more explorative surface individuals are more likely to colonise a markedly different habitat. However, we have to consider alternative explanations too. We found recently that potential colonists from the Malom Lake highly prefer surface food (decaying leaves) over cave food (bacterial mats); in fact, they spent the most time with feeding on surface food compared to other populations (Herczeg et al. 2022), implying that they were food-deprived ; hence, it is likely that they are starving in the cave. Therefore, it is possible that the detected trend for high behavioural activity is simply a result of starving individuals searching for the preferred surface food.
Unlike in other surface-type populations, behavioural activity of potential colonists from the Malom Lake did not decrease in the presence of light. As mentioned above, we possess no information regarding the time potential colonists spent in the cave; further, our experimental design did not allow us to test for genetic vs. environmental effects. Yet, it is very unlikely that this behavioural difference between colonists and other surface populations is the result of evolutionary (genetic) adaptation. Phenotypic plasticity (i.e. a genotype’s ability to develop or express alternative phenotypes in different environments; West-Eberhard, 2003) is a more plausible explanation. This behavioural pattern is likely not reflecting genetic adaptation to the cave environment, which should trigger a behavioural response similar to that found in the cave-adapted population.
Conclusions
Taken together, we found support for various links between exploratory behaviour and the colonisation of markedly new habitats. First, we found that following successful colonisation of a highly specialised and isolated cave habitat by A. aquaticus, mean exploratory behaviour of the cave population decreased. The result suggests that highly specialised and island-like habitats, like caves, can act as dispersal traps, where the benefits of explorativeness are negligible. Second, our results suggest that more explorative individuals are likely to enter the cave from the source surface population. This result implies that exploratory personality not only is linked to dispersal, but also determines the probability of colonising new habitats. Finally, we found an additional pattern that provides important details to the biology of A. aquaticus: surface A. aquaticus are not clearly diurnal. They are not only active in light, but also active in total darkness, and actually more active in dark than at light. This finding strengthens the field observation about high night activity of A. aquaticus (Andrikovics 1981) and also suggests that the species is exapted to cave life, explaining the species’ repeated successful colonisations of various caves in Europe (Prevorčnik et al. 2004; Konec et al. 2015). We have to note here that our results originate from a single, unique cave system; furthermore, we practically compared four populations (plus potential cave colonists from a surface population) and not two habitats. Thus, we cannot make generalisations about A. aquaticus behaviour in caves and it is reasonable to expect that different selection regimes in other cave systems might cause behaviour to evolve in different directions; further, we cannot exclude the possibility of stochastic events in trait evolution via founder effects or genetic drift (Wessel et al. 2013; Miller et al. 2020). However, as the Molnár János Cave population is isolated for at least 60,000 years from the closest surface populations (Pérez-Moreno et al. 2017), including the one in the directly connected Malom Lake, we still expect that any patterns where the cave population was clearly divergent from the three surface populations would result from adaptation to the cave environment.
Data availability
Data are available in the Electronic Supplementary Material.
References
Andrikovics S (1981) Further data to the daily migration of the larvae of aquatic insects. Opusc Zool Instituti Zoosystematici Oecologici Univ Budapestiensis 17–18:49–55
Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED (2012) Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol 23:960–969. https://doi.org/10.1093/beheco/ars059
Balázs G, Lewarne B, Herczeg G (2020) Extreme site fidelity of the olm (Proteus anguinus) revealed by a long-term capture–mark–recapture study. J Zool 1–7. https://doi.org/10.1111/jzo.12760
Balogh A, Ngo L, Zigler KS, Dixon G (2020) Population genomics in two cave-obligate invertebrates confirms extremely limited dispersal between caves. Sci Rep 10:17554. https://doi.org/10.1038/s41598-020-74508-9
Barton K (2009) Mu-MIn: multi-model inference. R Package Version 0.12.2/r18. http://r-forge.r-project.org/projects/mumin/
Baxter-Gilbert J, Riley JL, Whiting MJ (2019) Bold new world: urbanization promotes an innate behavioral trait in a lizard. Behav Ecol Sociobiol 73:105. https://doi.org/10.1007/s00265-019-2713-9
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models using lme4. J Stat Softw 67:1-48. https://doi.org/10.18637/jss.v067.i01
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783. https://doi.org/10.1016/j.anbehav.2008.12.022
Berczik Á (1956) Újabb hidrobiológiai vizsgálatok a Lukács gyógyfürdő Malom-taván. Állattani Közlemények 45:35–44
Berisha H, Horváth G, Fišer Ž, Balázs G, FišerC, Herczeg G (2022) Sex-dependent increase of movement activity in the freshwater isopod Asellus aquaticus following adaptation to a predator-free cave habitat . Curr Zool 1–8. https://doi.org/10.1093/cz/zoac063
Bertin A, David B, Cézilly F, Alibert P (2002) Quantification of sexual dimorphism in Asellus aquaticus (Crustacea: Isopoda) using outline approaches. Biol J Linn Soc 77:523–533. https://doi.org/10.1046/j.1095-8312.2002.00125.x
Bloor MC (2011) Dietary preference of Gammarus pulex and Asellus aquaticus during a laboratory breeding programme for ecotoxicological studies. Int J Zool 2011. https://doi.org/10.1155/2011/294394
Blumstein DT, Wey TW, Tang K (2009) A test of the social cohesion hypothesis: interactive female marmots remain at home. Proc R Soc B Biol Sci 276:3007–3012. https://doi.org/10.1098/rspb.2009.0703
Bodor P, Erőss A, Mádlné Szőnyi J, Kovács J (2015) A csapadék hatása a rózsadombi források utánpótlódási és megcsapolódási területén. Földtani Közlöny 145:385–395
Bókony V, Kulcsár A, Tóth Z, Liker A (2012) Personality traits and behavioral syndromes in differently urbanized populations of house sparrows (Passer domesticus). PLoS One 7. https://doi.org/10.1371/journal.pone.0036639
Borowsky R, Cohen D (2013) Genomic consequences of ecological speciation in Astyanax cavefish. PLoS One 8. https://doi.org/10.1371/journal.pone.0079903
Breed M, Moore J (2021) Animal behavior, 3rd edn. Academic Press, London
Brengdahl M (2016) Dispersive trait expression of Asellus aquaticus from a rare cave habitat. Dissertation. Linköping University
Careau V, Bininda-Emonds ORP, Thomas DW, Réale D, Humphries MM (2009) Exploration strategies map along fast-slow metabolic and life-history continua in muroid rodents. Funct Ecol 23:150–156. https://doi.org/10.1111/j.1365-2435.2008.01468.x
Chevaldonné P, Sket B, Marschal C, Lejeusne C, Calado R (2008) Improvements to the “Sket bottle”: a simple manual device for sampling small crustaceans from marine caves and other cryptic habitats. J Crustac Biol 28:185–188. https://doi.org/10.1651/07-2923R.1
Cote J, Clobert J, Brodin T, Fogarty S, Sih A (2010) Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos Trans R Soc Lond B Biol Sci 365:4065–4076. https://doi.org/10.1098/rstb.2010.0176
Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A (2010) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc Biol Sci 277:1571–1579. https://doi.org/10.1098/rspb.2009.2128
Cote J, Brodin T, Fogarty S, Sih A (2017) Non-random dispersal mediates invader impacts on the invertebrate community. J Anim Ecol 86:1298–1307. https://doi.org/10.1111/1365-2656.12734
Culver DC, Pipan T (2019) Biology of caves and other subterranean habitats, 2nd editio. Oxford University Press
Culver DC, Poulson TL (1971) Oxygen consumption and activity in closely related amphipod populations from cave and surface habitats. Am Midl Nat 85:74–84
Danielopol D, Rouch R (2005) Invasion, active versus passive. In: Culver D, White WB (eds) Encyclopedia of caves. st edn. Academic Press, Amsterdam, pp 305–310
Dember W, Richman C (1989) Spontaneous alternation behavior. Springer, New York
Dingemane NJ, Wolf M (2010) Recent models for adaptive personality differences: a review. Phil Trans R Soc B Biol Sci 365:3947–3958
Dingemanse NJ, Both C, van Noordwijk AJ, Rutten AL, Drent PJ (2003) Natal dispersal and personalities in great tits (Parus major). Proc R Soc B 270:741–747. https://doi.org/10.1098/rspb.2002.2300
Dobosy P, Sávoly Z, Óvári M, Mádl-Szőnyi J, Záray G (2016) Microchemical characterization of biogeochemical samples collected from the Buda Thermal Karst System, Hungary. Microchem J 124:116–120. https://doi.org/10.1016/j.microc.2015.08.004
Edelaar P, Siepielski AM, Clobert J (2008) Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution (n y) 62:2462–2472. https://doi.org/10.1111/j.1558-5646.2008.00459.x
Enyedi NT, Anda D, Borsodi AK, Szabó A, Pál SE, Óvári M, Márialigeti K, Kovács-Bodor P, Mádl-Szőnyi J, Makk J (2019) Radioactive environment adapted bacterial communities constituting the biofilms of hydrothermal spring caves (Budapest, Hungary). J Environ Radioact 203:8–17. https://doi.org/10.1016/j.jenvrad.2019.02.010
Erőss A, Mádl-Szőnyi J, Mindszenty A, Müller I (2006) Conclusions from a negative tracer test in the urban thermal karst area, Budapest, Hungary. In: Tellam J, Rivett M, Israfilov R (eds) Urban groundwater management and sustainability. Springer, pp 289–298
Eroukhmanoff F, Hargeby A, Arnberg NN, Hellgren O, Bensch S, Svensson EI (2009) Parallelism and historical contingency during rapid ecotype divergence in an isopod. J Evol Biol 22:1098–1110. https://doi.org/10.1111/j.1420-9101.2009.01723.x
Fišer Ž, Novak L, Luštrik R, Fišer C (2016) Light triggers habitat choice of eyeless subterranean but not of eyed surface amphipods. Naturwissenschaften 103:7. https://doi.org/10.1007/s00114-015-1329-9
Fišer Ž, Prevorčnik S, Lozej N, Trontelj P (2019) No need to hide in caves: shelter-seeking behavior of surface and cave ecomorphs of Asellus aquaticus (Isopoda: Crustacea). Zoology 134:58–65. https://doi.org/10.1016/j.zool.2019.03.001
Fraenkel G, Gunn D (1961) The orientation of animals: kineses, taxes and compass reactions. Dover Publications Inc, New York
Garamszegi LZ, Markó G, Herczeg G (2013) A meta-analysis of correlated behaviors with implications for behavioral syndromes: relationships between particular behavioral traits. Behavi Ecol 24:1068–1080. https://doi.org/10.1093/beheco/art033
Hasu T, Holmes J, Valtonen E (2007) Isopod (Asellus aquaticus) size and Acanthocephalan (Achantocephalus licii) infections. J Parasitol 93:450–457
Herczeg G, Hafenscher VP, Balázs G, Fišer Ž, Kralj- Fišer S, Horváth G (2020) Is foraging innovation lost following colonisation of a less variable environment? A case study in surface- vs. cave- dwelling Asellus aquaticus. Ecol Evol 10:5323–5331. https://doi.org/10.1093/comnet/xxx000
Herczeg G, Nyitrai V, Balázs G, Horváth G (2022) Food preference and food type innovation of surface‑ vs . cave‑dwelling waterlouse (Asellus aquaticus) after 60 000 years of isolation. Behav Ecol Sociobiol 76:1. https://doi.org/10.1007/s00265-021-03109-x
Hervant F, Renault D (2002) Long-term fasting and realimentation in hypogean and epigean isopods: a proposed adaptive strategy for groundwater organisms. J Exp Biol 205:2079–2087
Hervant F, Mathieu J, Barré H et al (1997) Comparative study on the behavioral, ventilatory, and respiratory responses of hypogean and epigean crustaceans to long-term starvation and subsequent feeding. Comp Biochem Physiol - A Physiol 118:1277–1283. https://doi.org/10.1016/S0300-9629(97)00047-9
Hervant F, Mathieu J, Durand J (2001) Behavioural, physiological and metabolic responses to long-term starvation and refeeding in a blind cave-dwelling (Proteus anguinus) and a surface-dwelling (Euproctus asper) salamander. J Exp Biol 204:269–281
Howarth FG (1980) The zoogeography of specialized cave animals: a bioclimatic model. Evolution (n y) 34:394–406
Hüppop K (2000) How do cave animals cope with the food scarcity in caves? In: Wilkens H, Culver DC, Humphreys FW (eds) Ecosystems of the world: subterranean ecosystems. Elsevier, Amsterdam, pp 159–188
Jacob S, Bestion E, Legrand D, Clobert J, Cote J (2015) Habitat matching and spatial heterogeneity of phenotypes: implications for metapopulation and metacommunity functioning. Evol Ecol 29:851–871. https://doi.org/10.1007/s10682-015-9776-5
Janzer W, Ludwig W (1952) Versuche zur Evolutorischen Entstehung der Höhlentiermerkmale. Zeitschriftung Für Indukt Abstammungs- Und Vererbungslehre 84:462–479
Juan C, Emerson BC (2010) Evolution underground: shedding light on the diversification of subterranean insects. J Biol 9. https://doi.org/10.1186/jbiol227
Konec M, Prevorčnik S, Sarbu SM, Verovnik R, Trontelj P (2015) Parallels between two geographically and ecologically disparate cave invasions by the same species, Asellus aquaticus (Isopoda, Crustacea). J Evol Biol 28:864–875. https://doi.org/10.1111/jeb.12610
Kuznetsova A, Brockhoff P, Christensen R (2016) lmerTest package: tests in linear mixed effects models, R package version 2.0–33. https://cran.r-project.org/package=lmerTest
Lafuente E, Lürig MD, Rövekamp M, Matthews B, Buser C, Vorburger C, Räsänen K (2021) Building on 150 years of knowledge: the freshwater isopod Asellus aquaticus as an integrative eco-evolutionary model system. Front Ecol Evol 9:748212. https://doi.org/10.3389/fevo.2021.748212
Lefébure T, Douady CJ, Malard F, Gibert J (2007) Testing dispersal and cryptic diversity in a widely distributed groundwater amphipod (Niphargus rhenorhodanensis). Mol Phylogenet Evol 42:676–686. https://doi.org/10.1016/j.ympev.2006.08.020
Lenth R (2019) emmeans: Estimated marginal means, aka least-squares means. R-project. https://github.com/rvlenth/emmeans
Mejía-Ortíz LM, Hartnoll RG, López-Mejía M (2006) Progressive troglomorphism of ambulatory and sensory appendages in three Mexican cave decapods. J Nat Hist 40:255–264. https://doi.org/10.1080/00222930600628382
Mettke-Hofmann C, Winkler H, Leisler B (2002) The significance of ecological factors for exploration and neophobia in parrots. Ethology 108:249–272. https://doi.org/10.1046/j.1439-0310.2002.00773.x
Miller TEX, Angert AL, Brown CD, Lee‐Yaw JA, Lewis M, Lutscher F, Marculis NG, Melbourne BA, Shaw AK, Szűcs M, Tabares O, Usui T, Weiss‐Lehman C, Williams JL (2020) Eco‐evolutionary dynamics of range expansion. Ecology 101:e03139. https://doi.org/10.1002/ecy.3139
Morse D (1980) Behavioral mechanisms in ecology. Harvard University Press
Myles-Gonzalez E, Burness G, Yavno S, Rooke A, Fox MG (2015) To boldly go where no goby has gone before: boldness, dispersal tendency, and metabolism at the invasion front. Behav Ecol 26:1083–1090. https://doi.org/10.1093/beheco/arv050
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Niemelä PT, Dingemanse NJ (2018) On the usage of single measurements in behavioural ecology research on individual differences. Anim Behav 145:99–105. https://doi.org/10.1016/j.anbehav.2018.09.012
O’Hara RB, Kotze DJ (2010) Do not log-transform count data. Methods Ecol Evol 1:118–122. https://doi.org/10.1111/j.2041-210x.2010.00021.x
Payton ME, Greenstone MH, Schenker N (2003) Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci 3. https://doi.org/10.1093/jis/3.1.34
Peck S, Finston D (1993) Galapagos Islands troglobites: the questions of tropical troglobites, parapatric distributions with eyed-sister-species, and their origin by parapatric speciation. Mem Biospéologie 20:19–37
Pérez-Moreno JL, Balázs G, Wilkins B, Herczeg G, Bracken-Grissom HD (2017) The role of isolation on contrasting phylogeographic patterns in two cave crustaceans. BMC Evol Biol 17:247. https://doi.org/10.1186/s12862-017-1094-9
Pérez-Moreno JL, Balázs G, Bracken-Grissom HD (2018) Transcriptomic insights into the loss of vision in Molnár János Cave’s crustaceans. Integr Comp Biol 58:452–464. https://doi.org/10.1093/icb/icy071
Porter ML, Crandall KA (2003) Lost along the way: the significance of evolution in reverse. Trends Ecol Evol 18:541–547. https://doi.org/10.1016/S0169-5347(03)00244-1
Prevorčnik S, Blejec A, Sket B (2004) Racial differentiation in Asellus aquaticus (L.) (Crustacea: Isopoda: Asellidae). Arch Für Hydrobiol 160:193–214. https://doi.org/10.1127/0003-9136/2004/0160-0193
R Developmental Core Team (2021) R: a language and environment for statistical computing. https://www.r-project.org/
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Rivera MAJ, Howarth FG, Taiti S, Roderick GK (2002) Evolution in Hawaiian cave-adapted isopods (Oniscidea: Philosciidae): vicariant speciation or adaptive shifts? Mol Phylogenet Evol 25:1–9. https://doi.org/10.1016/S1055-7903(02)00353-6
Romero A (1985) Cave colonization by fish: role of bat predation. Am Midl Nat 113:7–12
Romero A (2009) Cave biology: life in darkness, 1st edn. Cambridge University Press
Saltz JB (2019) Gene-environment correlation in humans: lessons from psychology for quantitative genetics. J Hered 110:455–466. https://doi.org/10.1093/jhered/esz027
Saltz JB, Bell AM, Flint J, Gomulkiewicz R, Hughes KA, Keagy J (2018) Why does the magnitude of genotype-by-environment interaction vary? Ecol Evol 8:6342–6353. https://doi.org/10.1002/ece3.4128
Schuett W, Delfs B, Haller R, Kruber S, Roolfs S, Timm D, Willmann M, Drees C (2018) Ground beetles in city forests: does urbanization predict a personality trait? PeerJ 6:e4360. https://doi.org/10.7717/peerj.4360
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. https://doi.org/10.1016/j.tree.2004.04.009
Sih A, Cote J, Evans M, Fogarty S, Pruitt J (2012) Ecological implications of behavioural syndromes. Ecol Lett 15:278–289. https://doi.org/10.1111/j.1461-0248.2011.01731.x
Spiegel O, Leu ST, Bull CM, Sih A (2017) What’s your move? Movement as a link between personality and spatial dynamics in animal populations. Ecol Lett 20:3–18. https://doi.org/10.1111/ele.12708
Stern DB, Breinholt J, Pedraza-Lara C, López-Mejía M, Owen CL, Bracken-Grissom H, Fetzner JW Jr, Crandall KA (2017) Phylogenetic evidence from freshwater crayfishes that cave adaptation is not an evolutionary dead-end. Evolution (n y) 71:2522–2532. https://doi.org/10.1111/evo.13326
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Thompson DJ, Manning JT (1981) Mate selection by Asellus (Crustacea:Isopoda). Behaviour 78:178–187
Tobler M (2009) Does a predatory insect contribute to the divergence between cave- and surface-adapted fish populations? Biol Lett 5:506–509. https://doi.org/10.1098/rsbl.2009.0272
Trontelj P, Douady CJ, Fišer C, Gilbert J, Gorički Š, Lefébure T, Sket B, Zakšek V (2009) A molecular test for cryptic diversity in ground water: how large are the ranges of macro-stygobionts? Freshw Biol 54:727–744. https://doi.org/10.1111/j.1365-2427.2007.01877.x
West-Eberhard MJ (2003) Developmental plasticity and evolution. 1st edn. Oxford University Press, New York
Wessel A, Hoch H, Asche M, von Rintelen T, Stelbrink B, Heck V, Stone FD, Howarth FG (2013) Founder effects initiated rapid species radiation in Hawaiian cave planthoppers. Proc Natl Acad Sci USA 110:9391-9396. https://doi.org/10.1073/pnas.1301657110
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We are grateful for Ned A. Dochtermann and two anonymous researchers for their valuable remarks which increased the quality of our paper substantially.
Funding
Open access funding was provided by Eötvös Loránd University. The study was funded by the National Research, Development and Innovation Office for international cooperation (SNN 125627). GeH also gained support from the Postdoctoral research grant of the National Research, Development and Innovation Office (PD 132041). GB was supported by the ÚNKP-20–4 New National Excellence Program of the Ministry of Innovation and Technology from the source of the National Research, Development and Innovation Office.
Author information
Authors and Affiliations
Contributions
GeH, GB, HB and GáH designed the study; GeH, KK, GB and HB performed the experiments and collected data; GeH, KK and VN analysed the data; GeH drafted the manuscript with the substantial contribution of GáH; all authors reviewed the manuscript and gave final approval for publication.
Corresponding author
Ethics declarations
Ethical approval
Experiments were performed according to the guide- lines of the Hungarian Act of Animal Care and Experimentation (1998, XXVIII, Sect. 243/1998), which conforms to the regulation of animal experiments by the European Union. At the end of the experiment, animals were euthanised and preserved in RNAlater for further research.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by N. A Dochtermann
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horváth, G., Kerekes, K., Nyitrai, V. et al. Exploratory behaviour divergence between surface populations, cave colonists and a cave population in the water louse, Asellus aquaticus. Behav Ecol Sociobiol 77, 15 (2023). https://doi.org/10.1007/s00265-022-03288-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03288-1