Abstract
Yawning is a complex behaviour linked to several physiological (e.g. drowsiness, arousal, thermoregulation) and social phenomena (e.g. yawn contagion). Being yawning an evolutionary well-conserved, fixed action pattern widespread in vertebrates, it is a valuable candidate to test hypotheses on its potential functions across the different taxa. The spotted hyaena (Crocuta crocuta), the most social and cooperative species of the Hyaenidae family, is a good model to test hypotheses on yawning correlates and significances. Through an accurate sequential analysis performed on a group of wild hyaenas, we found that yawning mainly occurred during an imminent behavioural state changing in both juveniles and adults and that seeing others’ yawn elicited a mirror response in the receiver, thus demonstrating that yawn contagion is present in this species. These results taken together suggest that yawning is linked to a behavioural state change of the yawner and that such change is caught by the observers that engage in a motor resonance phenomenon, yawn contagion, possibly effective in anticipating yawners’ motor actions. Although additional data are necessary to verify whether yawn contagion translates into subsequent motor convergence and alignment, our data suggest that both spontaneous and contagious yawning can be fundamental building blocks on the basis of animal synchronisation in highly social and cooperative species.
Significant statement
Yawning is pervasive in many animal species, including humans. It is considered as a polyfunctional cue that has a role in regulating social interactions. While several studies focussed on yawning functions in primates, a little amount of effort was devoted to exploring this behaviour in social carnivores. We monitored a group of wild spotted hyaenas (Crocuta crocuta), which is one of the most cooperative carnivore species. In both immature and adult subjects, we found that a subject frequently changed its behavioural state after spontaneously yawning and that seeing others’ yawn elicited a mirror response in the observer. Although additional data are necessary to verify whether yawn contagion translates into subsequent motor convergence and alignment, our data suggest that both spontaneous and contagious yawning can be fundamental building blocks on the basis of animal synchronisation in highly social and cooperative species.
Similar content being viewed by others
Introduction
Spontaneous yawning is a fixed action pattern consisting of long, deep inhalation, a brief peak with apnea, followed by a short exhalation (Walusinski and Deputte 2004; Guggisberg et al. 2010; Provine 2012; Gallup et al. 2016). It can also include active jaw gaping, eye closure, contraction of facial muscles, and passive jaw closure, often accompanied by neck stretching and head tilting and, sometimes, by limb and body stretching (pandiculation). Due to its complex combination of motor patterns in humans, Provine (2012) distinguished different types of a yawn (close-nose; clenched-teeth; sealed lips nose; eyes open), suggesting that a certain level of variation exists in the expression of the behaviour. Yawning is an evolutionary well-conserved pattern that is widespread in the different vertebrate lineages (fish, reptiles, birds, mammals) (Baenninger 1987; Massen et al. 2021). From a physiological viewpoint, yawning seems to be the expression of a behavioural state change linked to drowsiness, arousal and thermoregulation (Guggisberg et al. 2010; Krestel et al. 2018). In many vertebrate species, a yawn punctuates the switch from the awake to the sleepy phase (and vice versa) (e.g. ostriches, Struthio camelus australis, Sauer and Sauer 1967; human, Provine 2005; red hill salamanders, Phaeognathus hubrichti, Bakkegard 2017; South American sea lions, Otaria flavescens, Palagi et al. 2019a; African lions, Panthera leo, Casetta et al. 2021; dugongs, Dugong dugon, Enokizu et al. 2022; bottlenose dolphins, Tursiops truncatus, Enokizu et al. 2021; non-human primates, Zannella et al. 2021). In this context, yawning increases alertness, thus making subjects able to adjust their behaviour in response to sudden and unexpected situations (Provine 2005; Gallup 2022). Spontaneous yawning also varies according to the stimuli the subject receives from its social environment (Greco et al. 1993; Deputte 1994; Baenninger 1997; Provine 1997; Guggisberg et al. 2010). The arousal hypothesis suggests a link between yawning and the expression of an emotional state changing with a positive or negative valence depending on the information received from the social environment. Under such circumstances, yawning facilitates physiological/emotional homeostasis by functioning, for example, as a stress-releaser mechanism when the stimulus has a negative valence (Nazca booby birds, Sula granti, Liang et al. 2015; budgerigars, Melopsittacus undulatus, Miller et al. 2010; rats, Rattus norvegicus Moyaho and Valencia 2002; lemurs, Zannella et al. 2015; geladas, Theropithecus gelada Leone et al. 2014; macaques, Macaca spp. Zannella et al. 2017; South American sea lions, Palagi et al. 2019a).
In addition to the physiological functions, scholars have long hypothesised social functions for yawning (Deputte 1994; Guggisberg et al. 2010; Leone et al. 2014; Moyaho et al. 2017; Gallup 2022). The physiological underpinnings and the social aspects of the yawning phenomenon are often studied separately, even though they are strongly interconnected. Detecting yawns from conspecifics can elicit overt behavioural changes, such as yawn contagion, which is a reflexive matching action occurring when others’ yawns trigger the same pattern in the observers (Provine 1986, 2005; Palagi et al. 2009; Guggisberg et al. 2010). Being more evolutionary recent than spontaneous yawning, contagious yawning can be observed in those social species that show a high level of cohesion, social tolerance and cooperation (geladas, Theropithecus gelada; bonobos, Pan paniscus; lions, Panthera leo; see Palagi et al. 2020 and Gallup 2022 for an extensive review). For example, yawn contagion has not been found in lowland gorillas (Gorilla gorilla gorilla), a social species whose group formation relies more on spatial proximity than on social affiliation (Palagi et al. 2019b). Yawn contagion, together with other facial resonance phenomena, seems to foster the subsequent motor action synchronisation in a sort of domino effect, a phenomenon extremely adaptive in several social mammals (McDougall and Ruckstuhl 2018; Casetta et al. 2021). The behaviour is also sensitive to the relationship quality shared between interacting subjects: the higher the social closeness, the higher the probability of yawn contagion (Romero et al. 2014; Palagi et al. 2020; Kret and van Berlo 2021). The positive effect of social closeness on contagion works independently from the species the two interacting subjects belong to (Pedruzzi et al. 2022).
Being yawning is a highly conserved trait across vertebrates, it is a valuable candidate for cross-species comparisons. For this reason, testing hypotheses on new taxa, especially in wild populations, is necessary to reach a full picture of this puzzling phenomenon. Like several species of social mammals, spotted hyaenas are organised in fission–fusion societies (Drea and Frank 2003; Smith et al. 2007) which are characterised by a nepotistic dominance hierarchical system (Kruuk 1972; Tilson and Hamilton 1984; Frank 1986; Mills 1990; Wahaj et al. 2004). Despite the strict hierarchy, the species shows a high level of social flexibility which is guaranteed by social support, cooperative behaviour and coalitions (Stratford and Periquet 2019; Vullioud et al. 2019). The reciprocal support in rearing offspring (König1997), hunting and defending territories (Holekamp et al. 2007) is an important social tool to manage power asymmetries among individuals (Vullioud et al. 2019). For all these reasons, the spotted hyaena is a good candidate to explore the potential correlates of yawning and check for the possible presence of its contagiousness.
The most recent findings on primates suggest that, although it is a stereotyped behaviour, yawning can be spontaneously produced with a certain degree of variability (e.g. frequency, duration, morphology). It has been demonstrated that yawn variation can predict the different physiological causes on the basis of its emission (Vick and Paukner 2010; Zannella et al. 2021). For example, yawning rates can be age-dependent, with juveniles yawning less than adults (Giganti and Salzarulo 2010). Moreover, frequency, duration and motor execution are also context-dependent with shorter and more frequent yawns being performed during arousal situations in several primate groups (Deputte 1994; Zannella et al. 2021). In some species of social carnivores, yawning is not affected by age; it is mainly performed under resting conditions and is consistent in its duration and performance (lions, Casetta et al. 2021; South American sea lions, Palagi et al. 2019a, b). Although recent studies indicate that yawning serves as a cue rather than as a true signal (e.g. it does not seem to suffer an audience effect, Aychet et al. 2021), its social function can be expressed by its contagiousness that, ultimately, can lead to behavioural convergence in the actions following the contagion event (Palagi et al. 2020; Casetta et al. 2021; Gallup 2022). Although we expect low variability in frequency, duration and performance of spontaneous yawning in the spotted hyaena due to the highly cooperative propensity in hunting, rearing of offspring and territory defence (Holekamp et al. 2007; Smith et al. 2008; Drea and Carter 2009; Nolfo et al. 2021, 2022), we hypothesise that spontaneous yawning contains clues on the possible internal state of the performer and that it can function as a social cue. Specifically, we predict that in spotted hyaenas, yawning can mark an imminent behavioural state changing in the yawner and that seeing others’ yawn can elicit a mirror response in the receiver (yawn contagion).
Methods
The reserve and data collection
The observations (June–October 2019) were conducted at the Siyafunda Wildlife & Conservation (-24.15029S, 30.65742E; Greater Makalali Private Game Reserve, GMPGR, Limpopo, South Africa) covering an area characterised by the savannah biome with herbaceous plants, tall trees and bushes (Low and Rebelo 1996). The Makhutswi River, a tributary of the Olifants river, crosses the reserve. Spotted hyaenas were introduced in 1995, and their number in the GMPGR is unknown. By patrolling the reserve areas, GC, APN and the rangers identified four dens (Fig. 1) and individually recognised 64 subjects (14 cubs, five juveniles, 45 subadults/adults) by using scars, patches of missing fur and fur spots (Holekamp and Smale 1998; Holekamp et al. 1996). The identification of the subjects was also facilitated by using the pictures accumulated by the rangers of the reserve over the years. The subjects were intercepted by patrolling the various zones and dens known to be frequented by hyaenas. By tracking walks, both the observers and the rangers followed the animals’ tracks to the dens. During the period of data collection, the four active dens (Fig. 1) represented the observation spots for collecting videos on the lactating females, their cubs and all the visiting subjects. The number of males and females was unknown due to the difficulty to recognise sex in this species (Frank 1986). The observers collected data from vehicles to which the animals were well habituated. It was not possible to record data blind because our study involved focal animals in the field.
Spatial distribution of the dens at the Siyafunda Wildlife & Conservation (-24.15029S, 30.65742E; Greater Makalali Private Game Reserve, GMPGR, Limpopo, South Africa). The dots highlighted by the empty red circles and the camera-clips indicate the dens monitored for the present study (Lufafa 1; Lufafa 2; Pidwa South; H90)
The sessions to search for the animals ranged from two to three per day (Ndays = 57) and occurred during the following time slots: 05.00–11.00 am; 03.00–06.00 pm; 06.00–10.00 pm. Since it is not easy to spot the animals, despite our efforts, we were able to collect about 26 h of high-quality videos that were directly recorded by the observers with the aid of a 50 × optical zoom camera (Canon EOS 110D; Full HD Panasonic Lumix DC-FZ82) to ensure observations at long distances (up to 50 m). The use of two cameras allowed continuous video recording of all the visible subjects also when they scattered around the spot. The videos from the two cameras were perfectly matched thanks to their same time setting. The nocturnal videos (18.00–22.00 h) were collected with the aid of red illumination, never directed towards the animals but to the ground around (Finley 1959; Spoelstra et al. 2017). This procedure was mandatory to limit the disturbance as much as possible.
A total of 12 h of videos was also collected with the aid of camera traps (Ranger digital trail, BN056) located in front of the dens (about 10 m from the entrance). The camera traps were fixed on trees 1.5 m above the ground and covered a range of 5 m around the den hole. The cameras worked 24 h/day with no delay between consecutive videos (lasting from 40 to 60 s), and the motor sensor was set at its maximum. The 24 subjects (8 cubs, 2 juveniles, 14 subadults/adults) with at least 30 min of video recordings were included in the analyses (observation time: mean 109 ± 19 SE min) (see Table 1 for details). Cubs and juveniles were clustered as ‘immature subjects’ and subadults and adults as ‘mature subjects’.
Video analyses and operational definitions
A.P.N. and G.C. analysed the videos by VLC 2.1.5 and Jump-to-Time extension (accuracy = 0.02 s) and underwent a 30 h training period under the supervision of EP, who also checked for inter-observer reliability. EP randomly selected some sections of the data set (about 10 min of videos) and verified whether the behavioural items (yawning, sitting, standing, lying down, walking, feeding) were correctly scored. The inter-observer reliability procedure was repeated every 2 h of videos analysed. Cohen’s kappa values for each of the behavioural item never scored below 0.92.
Spontaneous yawn
We applied the all-occurrences sampling method to extract all the yawning events from the videos. A yawn started when a subject opened its mouth, sometimes protruding its tongue, while simultaneously inhaling deeply, until the mouth opening reached the acme, during which the teeth were exposed thanks to lip retraction. Mouth closing and air exhalation were more rapid than the mouth opening and inhalation phases. For each yawn, we recorded (1) yawner identity, (2) exact time and duration, (3) yawner behavioural state (sitting, standing, lying down, walking), (4) individuals that could see/not-see the yawn and (5) the context. Spontaneous yawns could occur under two contexts: resting and arousal (feeding/social tension). During the resting context, the subject was lying down while switching from a sleeping/awake condition and vice versa, and it could engage in affiliative behaviours with groupmates (e.g. muzzle licking). If an animal was involved in a feeding session (e.g. around a carcass), the context was labelled as arousal/feeding.
Via a frame-by-frame analysis of the 88 yawns that were visible in each phase of their motor sequence, we measured the duration of each yawn from the first frame in which the lips appeared parted to the last frame in which the lips appeared closed (Fig. 2). Since all yawns recorded were silent, only visual contagion was considered. A yawn was categorised as spontaneous if it was not preceded by other yawns in the 3-min time window or if it was preceded by another yawning event, but the subjects present in the camera did not have the possibility to perceive it.
Behavioural shifting
We defined four possible conditions in which a behavioural shift (lying down standing, standing walking, standing lying down, walking standing) could occur (C1 = control condition 1; SY = spontaneous yawning condition; C2 = control condition 2; BL = baseline condition). To be conservative as much as possible and for comparative reasons (see Casetta et al. 2021), for this analysis, we selected a 1-min time gap. The SY covered a 1-min time gap which was divided into two 30-s blocks around a spontaneous yawning event (Fig. 3). The C1 and C2 covered a 1-min block preceding and following the SY, respectively (Fig. 3). These control time windows adjacent to the time window with yawning (SY) guarantees that the social context is basically the same for each single event. As a further control, we introduced a baseline condition (BL) that was obtained by randomly selecting a 1-min block of observation from a different video, not including any yawning event.
The lower part of the figure illustrates the four conditions in which a behavioural shift could occur (C1 = control condition 1, blue; SY = spontaneous yawning condition, red; C2 = control condition 2, green; BL = baseline, yellow). Squares indicate the behaviours (black square – black square = no shift; black square – white square = yes shift), and arrows indicate the possible shift (black arrow = no shift; white arrow = shift; black/white arrow = the shift had to occur after the yawn at t). The upper part of the figure represents the alluvial plot showing the probability of a SHIFTING event in the four conditions (C1, green stream, Nstatechanging = 7; SY, red stream, Nstatechanging = 35; C2, blue stream, Nstatechanging = 11; BL, yellow stream, Nstatechanging = 16). (R package ‘ggalluvial’; Brunson and Read 2020)
For C1, SY, C2 and BL, we verified the presence/absence of a possible SHIFT in the behavioural state. A SHIFT was considered to be present when the subject passed from a given behavioural state to another one (lying down vs standing, standing vs walking, standing vs lying down, walking vs standing). In C1, C2 and BL, if the animal changed its behavioural state within the 1-min time gap, this situation was classified as SHIFT. To be classified as SHIFT, in SY, the animal had to change its behavioural state in the 30-s block after yawning (Fig. 3).
Yawn contagion
A yawn was classified as not seen when (1) the face of the potential receiver was rotated by 180° with respect to the first yawner (hereafter, trigger) or (2) a visual obstacle (e.g. vegetation, rocks) was present between the two subjects. A yawn was classified as seen when no visual obstacle was present, and the receiver had its eyes open and positioned in front of the face of the trigger. For each yawning event, we also counted how many immature and adult subjects were present and observable by the experimenters (Nimm; Nad). All yawns performed by subjects that had seen a yawn were yawn contagion events. A yawn performed at t0 increases the probability that the same subject yawned again at t(0+X) with X indicating the unit of time (autocorrelation). Miscoding a spontaneous yawn as a contagious event is less likely in the first 3 min after seeing a yawn than later (Campbell and Cox 2019), when autocorrelation is more probable. However, if autocorrelation occurred with the second yawner engaging in more than one yawning response, we counted just only the first response and discarded the successive ones. For comparative reasons, to record yawn contagion events, we adopted the 3-min criterion already used by previous studies (Palagi et al. 2014, 2019b; Romero et al. 2014; Yonezawa et al. 2016; Campbell and Cox 2019; Casetta et al. 2021; Gallo et al. 2021).
Statistics
To compare the hourly frequency of spontaneous yawning between the two age classes of individuals (matures vs immatures), we applied the Mann–Whitney exact U test (non-normal data: Kruskal–Wallis test P < 0.05).
MODEL yawn duration
To evaluate which variable affected the duration of each yawn event (response variable), we ran a linear mixed model (LMM) with a Gaussian distribution. The fixed factors were DAY/NIGHT, ranges of environmental temperatures (TEMP; https://www.timeanddate.com) (T, °C: 5–10; 11–15; 16–20; 21–25; > 25), camera traps/observers (CAM/OBS), age-class of the yawner (AGE) and resting/arousal CONTEXT. The random factor was the yawner’s identity. We compared the full model (including the random factor and DAY/NIGHT, TEMP, CAM/OBS, AGE and CONTEXT) against a null model that included only the random factor. No collinearity was found between the fixed factors (range VIFmin = 1.41; VIFmax = 3.01). The dispersion parameter was 1.007 (P = 0.928).
MODEL SHIFT
To evaluate if the presence of a yawn predicts a behavioural state shifting (SHIFT, response variable), we ran a GLMM with a binomial error distribution. The full model included the following variables: DAY/NIGHT, TEMP, CAM/OBS, AGE, CONTEXT and CONDITION (C1, SY, C2, BL). To test if the variable CONDITION had a significant effect on the response variable, we built a full model including the random and all the fixed factors. Then, we compared this model against a control model that included the random and all the fixed factors but the factor CONDITION. The random factor was the yawner’s identity. No collinearity was found between the fixed factors (range VIFmin = 1.02; VIFmax = 3.26). The dispersion parameter was 0.982 (P = 0.579).
MODEL YC
To test if seeing others’ yawn elicited a yawn response in the receiver (yawn contagion, YC), we ran a GLMM with a binomial error distribution. The response variable was the presence/absence of yawning in the subjects that were present in the video and that had or did not have the possibility to see the triggering yawn (presence/absence of response). The fixed factors were Yseen/Ynot_seen, DAY/NIGHT, CAM/OBS, AGEreceiver, CONTEXT, Nimm and Nad. To understand if the Yseen/Ynot_seen had a significant effect on the response variable, we built a full model including the random and all the fixed factors. Then, we compared this model against a control model that included the random and all the fixed factors but the factor Yseen/Ynot_seen. The trigger/receiver dyad identities were entered as random factors. No collinearity was found between the fixed factors (range VIFmin = 1.45; VIFmax = 3.74). The dispersion parameter was 0.014 (P = 1.00).
To run the three models, we used the R package glmmTMB 1.2.5042 (Brooks et al. 2017).
The likelihood ratio test (LRT; Dobson 2002) was applied to compare the full model with the control model (Forstmeier and Schielzeth 2011). The LRT was also employed to test the significance of the fixed factors by using the function Anova (R package car 3.0e10; Fox and Weisberg 2011). To exclude the occurrence of collinearity among predictors, we examined the variance inflation factors (VIF; Fox 2015) with the R package performance of 0.4.4 (Lüdecke et al. 2020). Model fit and overdispersion were verified by using the ggthemes function (Arnold 2019). The marginal R2 (representing the variance explained by fixed factors only) and the conditional R2 (representing the variance explained by the entire model including both fixed and random factors; Nakagawa et al. 2017) were calculated via the R package MuMIn 1.43.17 (Barton 2020). Then, we used the ‘confint(x)’ function to interpret the estimated effects in the model as relative odds ratios. Relative odds ratio (i.e. the expected odds change for one unit increase in the explanatory variable when the remaining variables are set to their reference category) was used to evaluate the magnitude of the estimated effects. All analyses were performed using R 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org).
Results
Descriptive results
Overall, 79 spontaneous yawns from 13 out of 24 subjects were recorded. See Table 1 for additional details on the data collection. Six out of 20 subjects (30% of the subjects) that had the possibility to perceive a yawning stimulus engaged in yawn contagion (N = 9 events within 3 min after perceiving others’ yawns).
Effect of age on spontaneous yawning
Neither immature nor adult subjects have ever emitted vocalised yawns. The frequency of spontaneous yawns (number of yawns of A/minutes of videos of A) performed by mature and immature subjects showed no significant difference (Mann–Whitney exact test: U = 47.00, Nmatures = 14, Nimmatures = 10, P = 0.169; meanmatures = 0.0345 ± 0.0239 SE; meanimmatures = 0.0201 ± 0.0327 SE). Additional details are reported in Table 1.
Variable effecting yawn duration (MODELyawn duration)
No statistical difference was found between the full model (including the random factor and the factors DAY/NIGHT, TEMP, CAM/OBS, AGE and CONTEXT) and the null model (including only the random factor) (χ2 = 11.634, df = 8, P = 0.168). The mean duration of each yawning event was 2.67 s (± 0.79 SE) for the mature and 1.92 s (± 0.62 SE) for the immature subjects.
The presence of a yawning event predicts a SHIFT (MODEL SHIFT)
We found a significant difference between the full (including random and the factors DAY/NIGHT, TEMP, CAM/OBS, AGE, CONTEXT and CONDITION) and the control model (including the random and all the fixed factors but the factor CONDITION) (χ2 = 33.149, df = 3, P < 0.001). The variable CONDITION significantly affected the SHIFT response variable (Table 2). The Tukey post-hoc test revealed that SHIFT occurred more frequently during SY than during C1, C2 and BL (t-ratioC1 vs SY = − 4.638, df = 298, P < 0.001; t-ratioC1 vs C2 = − 1.002, df = 298, P = 0.748; t-ratioC1 vs BL = − 2.111, df = 298, P = 0.152; t-ratioSY vs C2 = 4.050, df = 298, P < 0.001; t-ratioSY vs BL = 3.022, df = 298, P = 0.014; t-ratioC2 vs BL = − 1.192, df = 298, P = 0.632) (Fig. 3). The percentages of the behavioural shits recorded in the SY condition were lying down vs standing (8.57%), standing vs walking (48.57%), standing vs lying down (17.14%) and walking vs standing (25.71%).
Contagious yawning (MODEL YC)
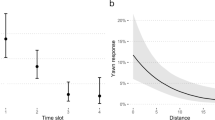
The full model (including the random and the factors DAY/NIGHT, CAM/OBS, AGEreceiver, CONTEXT, Nimm, Nad and Yseen/Ynot_seen,) significantly differed from the control model (including the random and all the fixed factors but the factor Yseen/Ynot_seen) (χ2 = 12.817, df = 1, P < 0.001). The variable Yseen/Ynot_seen had a significant effect on yawn contagion (Table 3) with the likelihood of yawn response higher in the Yseen than in Ynot_seen condition (Fig. 4). The latency of the yawning emission after seeing others’ yawns was 20.21 ± 6.74 s (mean ± SE).
Discussion
In wild spotted hyaenas, spontaneous yawning seems to be independent from the age and some features of the physical environment (e.g. temperatures, night/day). Neither the presence of the observer (camera traps/observer) nor the competitive context (resting vs feeding/aggression) affected both the occurrence and duration of spontaneous yawning (MODELyawn duration). However, since the absence of evidence is not the evidence of absence, these findings need to be taken with caution due to the small sample size and the variation represented by the variables.
Interestingly, no difference was also found in the overall frequency of yawning and its duration between immature and adult subjects. Therefore, as it occurs in other social carnivore species, yawning seems to be consistent in terms of its rates, duration and context throughout the entire life span, suggesting that this phenomenon is a well-conserved ontogenetic trait in wild spotted hyaenas. It is worth to note that similar findings were also obtained for another African species of social carnivore (Panthera leo, Casetta et al. 2021). By expanding the dataset to a larger number of subjects and a larger number of species of social carnivores, in the future, we will be able to verify the hypothesis of developmental consistency of spontaneous yawning behaviour and do some generalizations about the ontogenetic traits of the phenomenon in this taxon. Yawns seem to be more flexible in primates that are characterised by a high complexity in their facial musculature (Davila-Ross and Palagi 2022). In this mammalian order, the behaviour can vary in frequency, duration and motor execution as a function of the age of the subjects (Deputte 1994; Leone et al. 2014) and the contexts in which the behaviour occurs (Zannella et al. 2021). Further data on spontaneous yawning variability in both social carnivore and primate species are needed to understand if the phenomenon actually differs in these two lineages of mammals.
We found that in our study subjects, a behavioural shifting occurred more frequently after the emission of a spontaneous yawn, thus suggesting that the expression of the behaviour is predictive of the imminent changing in the state of the subject (MODELSHIFT; Table 2; Fig. 3). In a study involving some experimental trials on olfactory behaviour, Drea et al. (2002) found that captive hyaenas increased their levels of yawning (together with other self-directed behaviours) during periods of arousal provoked by the presence of a carrion odour. Under such condition, animals spent less time in resting by increasing their motor and social activity. These results are only apparently in contrast with ours. An increase in behavioural activity could mean a higher number of behavioural shifts that, in turn, can translate into a higher number of yawns. All these findings taken together indicate that the spontaneous emission of a yawn can be driven by a variety of endogenous factors translating into a modification of the behavioural activities. By analysing spontaneous yawning in white-cheeked mangabeys (Cercocebus albigena) and long-tailed macaques (Macaca fascicularis), Deputte (1994) postulated that the phenomenon was temporally associated with a change in the behavioural state of the subject (e.g. from rest to activity and after social interactions). Further data supporting this view were obtained by the comparison of the level of yawning between two sympatric species of wild lemurs. The omnivorous Lemur catta is characterised by a more dynamic lifestyle than the folivorous Propithecus verreauxi, with the former scoring a higher number of behavioural shifting events per unit of time than the latter, characterised by long periods of inactivity. Similarly, L. catta scores higher rates of yawning than P. verreauxi. This correlational evidence led the authors to suggest that the entity of the spontaneous yawning phenomenon could be linked to the level of behavioural activity of each species (Zannella et al. 2015). Another correlational study on chimpanzees also indicates that yawning can be a reliable indicator of behavioural activity in this great ape (Vick and Paukner 2010). Our data on hyaenas provide direct evidence of the strict temporal linkage between yawning and behavioural state changing, thus confirming in a cooperative carnivore the correlational data obtained for primates.
In wild hyaenas, seeing others’ yawns increased the probability to respond with a yawn in the observer independently from the age and the number of subjects, the environmental or context condition (Table 3; Fig. 4). Therefore, we can reasonably suppose that through yawn contagion, wild hyaenas are able to rapidly catch the imminent changes in others’ behaviour (a latency of about 20 s for a contagion event). Unfortunately, due to the limited sample size (N = 9 contagious yawns) and the difficulty to identify the sex of the subjects in the wild (Frank 1986), it was not possible to evaluate if responding with a yawn to others’ yawns increased the probability of motor synchrony between groupmates and especially in response to the dominant females which cover the leading role in this species. However, the recent findings on a large dataset obtained on another African social carnivore (Panthera leo, Casetta et al. 2021) make this hypothesis plausible also for wild spotted hyaenas. In wild lions, Casetta et al. (2021) did not investigate if a yawn actually occurred in coincidence with a changing in the behavioural status of the first yawner, although they demonstrated that being infected by a companion’s yawn provoked an alignment of the activities between the first and the second yawner. Considering the high levels of cohesion and cooperation typical of spotted hyaenas, we can reasonably hypothesise that yawn contagion can be an adaptive phenomenon also in this species and that it could help synchronise activities, especially between females due to their leading role in the groups (Stratford and Périquet 2019; Vullioud et al. 2019). This hypothesis would be worth to be investigated in the future by enlarging the datasets both on males and females and embracing the different subgroups deriving from the fission–fusion events typical of the species (Strauss et al. 2021). Due to the fluidity of their social systems, hyaenas can select companions to associate with. It could be extremely interesting to evaluate if such a choice could also be linked to the events of yawn contagion, possibly leading to behavioural synchrony.
Data availability
Raw data are available and submitted as supplementary material.
References
Arnold JB (2019) ggthemes: extra themes, scales and geoms for ‘ggplot2’. R package version 4(0), https://jrnold.github.io/ggthemes/
Aychet J, Blois-Heulin C, Palagi E, Lemasson A (2021) Facial displays in red-capped mangabeys (Cercocebus torquatus): repertoire, social context, and potential intentionality. J Comp Psychol 135:98–113. https://doi.org/10.1037/com0000252
Baenninger R (1997) On yawning and its functions. Psychon B Rev 4:198e207. https://doi.org/10.3758/BF03209394
Baenninger R (1987) Some comparative aspects of yawning in Betta splendens, Homo sapiens, Panthera leo and Papio sphinx. J Comp Psychol 101:349–354. https://doi.org/10.1037/0735-7036.101.4.349
Bakkegard KA (2017) Yawning by Red Hills salamanders (Phaeognathus hubrrichti) at their burrow entrance. Herpetol Rev 48:32–36
Bartoń K (2020) MuMIn: multi-model inference. R package, version 1.43.17, https:// CRAN.R-project.org/package=MuMIn
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zeroinflated generalized linear mixed modeling. R J 9:378–400
Brunson JC, Read QD (2020) “ggalluvial: alluvial plots in ‘ggplot2’.” R package, version 0.12.3, http://corybrunson.github.io/ggalluvial/
Campbell MW, Cox CR (2019) Observational data reveal evidence and parameters of contagious yawning in the behavioral repertoire of captive-reared chimpanzees (Pan troglodytes). Sci Rep 9:13271. https://doi.org/10.1371/journal.pone.0018283
Casetta G, Nolfo AP, Palagi E (2021) Yawn contagion promotes motor synchrony in wild lions, Panthera leo. Anim Behav 174:149–159. https://doi.org/10.1016/j.anbehav.2021.02.010
Deputte BL (1994) Ethological study of yawning in primates. I. Quantitative analysis and study of causation in two species of Old World monkeys (Cercocebus albigena and Macaca fascicularis). Ethology 98:221–245. https://doi.org/10.1111/j.1439-0310.1994.tb01073.x
Dobson AJ (2002) An introduction to generalized linear models, 2nd edn. Chapman and Hall/CRC Press, Boca Raton, FL
Drea CM, Carter AN (2009) Cooperative problem solving in a social carnivore. Anim Behav 78:967–977. https://doi.org/10.1016/j.anbehav.2009.06.030
Drea CM, Frank LG (2003) The social complexity of spotted hyenas. In: de Waal FBM, Tyack PL (eds) Animal social complexity: intelligence, culture, and individualized societies. Harvard University Press, Cambridge, MA, pp 121–148
Drea CM, Vignieri SN, Cunningham SB, Glickman SE (2002) Responses to olfactory stimuli in spotted hyenas (Crocuta crocuta): II. Investigation of environmental odors and the function of rolling. J Comp Psychol 116:331–341. https://doi.org/10.1037/0735-7036.116.4.331
Enokizu A, Morisaka T, Murakami K, Sakurai N, Ueda N, Yoshioka M (2021) Yawn-like behavior in captive common bottlenose dolphins (Tursiops truncatus). Behav Process 189:104444. https://doi.org/10.1016/j.beproc.2021.104444
Enokizu A, Morisaka T, Handa Y, Yoshioka M (2022) Observation of yawn-like behavior in a dugong (Dugong dugon). J Ethol 40:103–108. https://doi.org/10.1007/s10164-021-00732-z
Finley RB (1959) Observation of nocturnal animals by red light. J Mammal 40:591–594. https://doi.org/10.2307/1376280
Forstmeier W, Schielzeth H (2011) Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol 65:47–55. https://doi.org/10.1007/s00265-010-1038-5
Fox J (2015) Applied regression analysis and generalized linear models. Sage, Thousand Oaks, CA
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks, CA
Frank LG (1986) Social organization of the spotted hyena Crocuta Crocuta. II Dominance and Reproduction Anim Behav 34:1510–1527
Gallo A, Zanoli A, Caselli M, Palagi E, Norscia I (2021) First evidence of yawn contagion in a wild monkey species. Sci Rep 11:17957. https://doi.org/10.1038/s41598-021-96423-3
Gallup AC (2022) The causes and consequences of yawning in animal groups. Anim Behav 187:209–219. https://doi.org/10.1016/j.anbehav.2022.03.011
Gallup AC, Church AM, Pellegrino AJ (2016) Yawn duration predicts brain weight and cortical neuron number in mammals. Biol Lett 12:20160545
Giganti F, Salzarulo P (2010) Yawning throughout life. In: Walusinski O (ed.) The mystery of yawning in physiology and disease. S. Karger AG, Basel Switzerland, pp 26–31
Greco M, Baenninger R, Govern J (1993) On the context of yawning: when, where, and why? Psychol Rec 43:175–183
Guggisberg AG, Mathis J, Schnider A, Hess CW (2010) Why do we yawn? Neurosci Biobehav Rev 34:1267–1276. https://doi.org/10.1016/j.neubiorev.2010.03.008
Holekamp KE, Smale L (1998) Behavioral development in the spotted hyena. Bioscience 48:997–1005
Holekamp KE, Smale L, Szykman M (1996) Rank and reproduction in the female spotted hyaena. J Reprod Fertil 108:229–237
Holekamp KE, Sakai ST, Lundrigan BL (2007) Social intelligence in the spotted hyena (Crocuta crocuta). Phil Trans R Soc B 362:523–538. https://doi.org/10.1098/rstb.2006.1993
König B (1997) Cooperative care of young in mammals. Naturwissenschaften 84:95–104. https://doi.org/10.1007/s001140050356
Krestel H, Bassetti CL, Walusinski O (2018) Yawning-Its anatomy, chemistry, role, and pathological considerations. Prog Neurobiol 161:61–78. https://doi.org/10.1016/j.pneurobio.2017.11.003
Kret ME, van Berlo E (2021) Attentional bias in humans toward human and bonobo expressions of emotion. Evol Psychol 19:14747049211032816. https://doi.org/10.1177/14747049211032816
Kruuk H (1972) The spotted hyena: a study of predation and social behavior. University of Chicago Press, Chicago, IL
Leone A, Ferrari P, Palagi E (2014) Different yawns, different functions? Testing social hypotheses on spontaneous yawning in Theropithecus gelada. Sci Rep 4:4010. https://doi.org/10.1038/srep04010
Liang AC, Grace JK, Tompkins EM, Anderson DJ (2015) Yawning, acute stressors, and arousal reduction in Nazca booby adults and nestlings. Physiol Behav 140:38–43. https://doi.org/10.1016/j.physbeh.2014.11.029
Low AB, Rebelo AG (1996) Vegetation of South Africa, Lesotho and Swaziland. Department of Environmental Affairs and Tourism, Pretoria, South Africa
Lüdecke D, Makowski D, Waggoner P (2020) Performance: assessment of regression models performance, version 0.4.4. R package, https://CRAN.R-project.org/package=performance
Massen JJ, Hartlieb M, Martin JS et al (2021) Brain size and neuron numbers drive differences in yawn duration across mammals and birds. Commun Biol 4:503. https://doi.org/10.1038/s42003-021-02019-y
McDougall PL, Ruckstuhl KE (2018) Vigilance behaviour is more contagious when chewing stops: examining the characteristics of contagious vigilance in bighorn sheep. Behav Ecol Sociobiol 72:143. https://doi.org/10.1007/s00265-018-2536-0
Miller ML, Gallup AC, Vogel AR, Clark AB (2010) Handling stress initially inhibits, but then potentiates yawning in budgerigars (Melopsittacus undulatus). Anim Behav 80:615–619. https://doi.org/10.1016/j.anbehav.2010.05.018
Mills MGL (1990) Kalahari hyaenas: the behavioural ecology of two species. Unwin Hyman, London, UK
Moyaho A, Valencia J (2002) Grooming and yawning trace adjustment to unfamiliar environments in laboratory Sprague-Dawley rats (Rattus norvegicus). J Comp Psychol 116:263–269. https://doi.org/10.1037/0735-7036.116.3.263
Moyaho A, Flores Urbina A, Monjaraz Guzman E, Walusinski O (2017) Yawning: a cue and a signal. Heliyon 3:e00437. https://doi.org/10.1016/j.heliyon.2017.e00437
Nakagawa S, Johnson PCD, Schielzeth H (2017) The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed effects models revisited and expanded. J R Soc Interface 14:20170213
Nolfo AP, Casetta G, Palagi E (2021) Play fighting in wild spotted hyaenas: like a bridge over the troubled water of a hierarchical society. Anim Behav 180:363–373. https://doi.org/10.1016/j.anbehav.2021.07.012
Nolfo AP, Casetta G, Palagi E (2022) Visual communication in social play of a hierarchical carnivore species: the case of wild spotted hyenas. Curr Zool 68:411–422. https://doi.org/10.1093/cz/zoab076
Palagi E, Leone A, Mancini G, Ferrari PF (2009) Contagious yawning in gelada baboons as a possible expression of empathy. P Natl Acad Sci USA 106:19262–19267. https://doi.org/10.1073/pnas.0910891106
Palagi E, Norscia I, Demuru E (2014) Yawn contagion in humans and bonobos: emotional affinity matters more than species. PeerJ 2:e519. https://doi.org/10.7717/peerj.519
Palagi E, Guillén-Salazar F, Llamazares-Martín C (2019a) Spontaneous yawning and its potential functions in South American sea lions (Otaria flavescens). Sci Rep 9:17226. https://doi.org/10.1038/s41598-019-53613-4
Palagi E, Norscia I, Cordoni G (2019b) Lowland gorillas (Gorilla gorilla gorilla) failed to respond to others’ yawn: experimental and naturalistic evidence. J Comp Psychol 133:406–416. https://doi.org/10.1037/com0000175
Palagi E, Celeghin A, Tamietto M, Winkielman P, Norscia I (2020) The neuroethology of spontaneous mimicry and emotional contagion in human and non-human animals. Neurosci Biobehav Rev 111:149–165. https://doi.org/10.1016/j.neubiorev.2020.01.020
Pedruzzi L, Aychet J, Le Vern L, Maglieri V, Rossard A, Lemasson A, Palagi E (2022) Familiarity modulates both intra- and interspecific yawn contagion in red-capped mangabeys. Sci Rep 12:11138. https://doi.org/10.1038/s41598-02215395-0
Provine RR (1986) Yawning as a stereotyped action pattern and releasing stimulus. Ethology 72:109–122. https://doi.org/10.1111/j.1439-0310.1986.tb00611.x
Provine RR (1997) Yawns, laughs, smiles, tickles, and talking: naturalistic and laboratory studies of facial action and social communication. In: Russell JA, Fernandez Dols JM (eds) The psychology of facial expression. Cambridge University, Cambridge, UK, pp 158–175
Provine RR (2005) Yawning. Am Sci 93:532–539. https://doi.org/10.1511/2005.56.980
Provine RR (2012) Curious behavior: yawning, laughing, hiccupping, and beyond. Belknap/Harvard University Press, Cambridge, MA
Romero T, Ito M, Saito A, Hasegawa T (2014) Social modulation of contagious yawning in wolves. PLoS ONE 9:e105963. https://doi.org/10.1371/journal.pone.0105963
Sauer EG, Sauer EM (1967) Yawning and other maintenance activities in the South African ostrich. Auk 84:571–587
Smith JE, Memenis SK, Holekamp KE (2007) Rank-related partner choice in the fission-fusion society of the spotted hyena (Crocuta crocuta). Behav Ecol Sociobiol 61:753–765. https://doi.org/10.1007/s00265-006-0305-y
Smith JE, Kolowski JM, Graham KE, Dawes SE, Holekamp KE (2008) Social and ecological determinants of fission-fusion dynamics in the spotted hyaena. Anim Behav 76:619–636. https://doi.org/10.1016/j.anbehav.2008.05.001
Spoelstra K, van Grunsven RHA, Ramakers JJC, Ferguson KB, Raap T, Donners M, Visser ME (2017) Response of bats to light with different spectra: light-shy and agile bat presence is affected by white and green, but not red light. Proc R Soc B 284:20170075. https://doi.org/10.1098/rspb.2017.0075
Stratford K, Périquet S (2019) Dyadic associations reveal clan size and social network structure in the fission-fusion society of spotted hyaenas. Afr J Ecol 58:182–192
Strauss ED, Jensen FH, Gersick AS, Thomas M, Holekamp KE, Strandburg-Peshkin A (2021) Daily ranging and den usage patterns structure fission-fusion dynamics and social associations in spotted hyenas. bioRxiv, https://doi.org/10.1101/2021.10.01.462772
Tilson RT, Hamilton WJ (1984) Social dominance and feeding patterns of spotted hyaenas. Anim Behav 32:715–724
Vick SJ, Paukner A (2010) Variation and context of yawns in captive chimpanzees (Pan troglodytes). Am J Primatol 72:262–269. https://doi.org/10.1002/ajp.20781
Vullioud C, Davidian E, Wachter B, Rousset F, Courtiol A, Höner OP (2019) Social support drives female dominance in the spotted hyaena. Nat Ecol Evol 3:71–76. https://doi.org/10.1038/s41559-018-0718-9
Wahaj SA, Van Horn RC, Van Horn TL, Dreyer R, Hilgris R, Schwarz J, Holekamp KE (2004) Kin discrimination in the spotted hyena (Crocuta crocuta): nepotism among siblings. Behav Ecol Sociobiol 56:237–247
Walusinski O, Deputte BL (2004) The phylogeny, ethology and nosology of yawning. Rev Neurol 160:1011–1021. https://doi.org/10.1016/S0035-3787(04)71138-8
Yonezawa T, Sato K, Uchida M, Matsuki N, Yamazaki A (2016) Presence of contagious yawning in sheep. Anim Sci J 88:195–200. https://doi.org/10.1111/asj.12681
Zannella A, Norscia I, Stanyon R, Palagi E (2015) Testing yawning hypotheses in wild populations of two strepsirrhine species: Propithecus verreauxi and Lemur catta. Am J Primatol 77:1207–1215. https://doi.org/10.1002/ajp.22459
Zannella A, Stanyon R, Palagi E (2017) Yawning and social styles: different functions in tolerant and despotic macaques (Macaca tonkeana and Macaca fuscata). J Comp Psychol 131:179–188. https://doi.org/10.1037/com0000062
Zannella A, Stanyon R, Maglieri V, Palagi E (2021) Not all yawns tell the same story: the case of Tonkean macaques. Am J Primatol 83:e23263. https://doi.org/10.1002/ajp.23263
Acknowledgements
We thank the Siyafunda Wildlife and Conservation, especially the director Michael Job for his kind support and hospitality during the data collection. We also thank the rangers who provided invaluable help in tracking animals (in alphabetical order): Sam Adams, Chaz Domijan, Kai Harris, Emma Jenkins, Jelle Linssen, Kayla McClelland, Lukas Schefer and Derek Smith. We wish to thank Veronica Maglieri, Luca Pedruzzi and Anna Zanoli for their help in statistics and graphical outputs. Finally, we wish to thank the anonymous reviewers for their accurate revision.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. This study was supported by the University of Pisa and private funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the University of Pisa (Animal Care and Use Board) (Italy). Since the study was purely observational, the committee waived the need for a permit. The study was conducted with no manipulation of animals. All applicable international, national and/or institutional guidelines for the use of animals were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by C. Soulsbury.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 3809 KB)
Supplementary file2 (MP4 71375 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Casetta, G., Nolfo, A.P. & Palagi, E. Yawning informs behavioural state changing in wild spotted hyaenas. Behav Ecol Sociobiol 76, 152 (2022). https://doi.org/10.1007/s00265-022-03261-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03261-y