Abstract
Distinct behaviours can co-vary within individuals. As such, the magnitude of certain behaviours may be partly predicted by other behaviours, rather than the environment. This can constrain behaviours, potentially reducing behavioural variability. Pre-copulatory sexual cannibalism, the consumption of potential mates before copulation, can lead to females remaining unmated, particularly if males are rare. One possible explanation for the persistence of pre-copulatory cannibalism is that sexual cannibalism is correlated with high levels of aggression towards prey. Here, we test this in two species of praying mantis: the highly cannibalistic Miomantis caffra and the less cannibalistic Orthodera novaezealandiae. If cannibalism in M. caffra is linked to aggression towards prey, we predicted that (1) M. caffra would be more aggressive towards prey than O. novaezealandiae, (2) female M. caffra would be more aggressive than males, (3) aggression towards prey would be correlated across juvenile and adult instars for M. caffra but not O. novaezealandiae, and (4) aggression towards prey would be associated with a propensity for sexual cannibalism among individual M. caffra. We found evidence supporting predictions one and two, but not predictions three and four. Surprisingly, aggression was shown to be repeatable and correlated across instars for O. novaezealandiae but not M. caffra. Our results suggest sexual cannibalism is not a product of behavioural co-variation, even in clades where sexual cannibalism is common. This suggests that sexual cannibalism evolves due to the direct benefits it brings to females, rather than being a by-product of high aggression towards heterospecific prey.
Significance statement
In some animals, different behaviours co-vary within individuals. This may lead to the emergence of costly behaviours and reduce behavioural plasticity. It is theorized that pre-copulatory cannibalism is a costly behavioural by-product of selection for high levels of aggression towards prey. However, there are very few studies that explicitly test this. Here, we provide a behavioural comparison between two species of praying mantis that vary in their propensity to cannibalize and tested whether general aggression is linked cannibalism. We found that aggression towards prey in adults can be linked to juvenile aggression but not a propensity for cannibalism. Although cannibalism rates were higher in the species that was more aggressive towards prey, aggression towards prey was not linked to cannibalism within individuals. This suggests that pre-copulatory cannibalism is not a behavioural by-product but a result of direct selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence from certain species suggests that some behaviours are consistent within individuals across ecological contexts and correlate with different behaviours among individuals (Sih et al. 2004). This has the potential to restrict behavioural variation within individuals (Pruitt et al. 2008), which may have implications for species’ ecology (Pruitt and Riechert 2012). For example, if co-variation between behaviours reduces behavioural plasticity (Sih et al. 2012), this may reduce how effectively individuals can modify behaviour in the face of environmental perturbations. Aggression has been studied extensively with respect to consistency within individuals and covariant behaviours. Individual variation in aggression is known to be consistent across developmental and environmental contexts in certain species (Arnqvist and Henriksson 1997; Dingemanse et al. 2007) and can correlate with a variety of other behaviours that determine fitness (Smith and Blumstein 2008). For example, some evidence suggests that aggressive individuals are better dispersers (Dingemanse et al. 2003; Cote et al. 2010), bolder in the presence of predators (Huntingford 1976; Johnson and Sih 2005) and more territorial (Riechert and Hedrick 1993). Studies across several spider families have also shown that aggression towards prey is positively correlated with a propensity for pre-copulatory cannibalism: the killing and eating of potential mates prior to copulation (Arnqvist 1992; Riechert and Hedrick 1993; Arnqvist and Henriksson 1997; Johnson and Sih 2005; Pruitt et al. 2008; Rabaneda-Bueno et al. 2014).
Pre-copulatory cannibalism has been observed in several species of predatory invertebrates (Elgar 1992) but is most commonly reported in praying mantids (Barry et al. 2009; Walker and Holwell 2015) and spiders (Elgar and Nash 1988; Wilder and Rypstra 2008). Pre-copulatory cannibalism has the potential to be maladaptive for both sexes because males are killed and females increase their risk of dying as a virgin. As well as the individual-level costs, pre-copulatory cannibalism has the potential to reduce population growth rate and increase population extinction risk (Fisher et al. 2018). Currently, there are three main hypotheses for why pre-copulatory sexual cannibalism persists in nature: (1) adaptive foraging—females devour males they encounter in order to gain essential nutrients for egg production (Hurd et al. 1994; Barry et al. 2008; Roggenbuck et al. 2011); (2) mate choice—sexual cannibalism represents an extreme form of mate choice in which non-preferred males are devoured to prevent copulation (Hebets 2003; Persons and Uetz 2005; for a review see: Prenter et al. 2006); (3) aggressive spillover—adult female aggression towards conspecific males is a by-product of strong selection for juvenile aggression (Arnqvist 1992; Arnqvist and Henriksson 1997; Johnson and Sih 2005). As such, the aggressive spillover hypothesis (ASH) suggests that even though pre-copulatory sexual cannibalism may be costly for females, this cost can be offset by the benefits of high juvenile feeding rates.
If juvenile and adult aggression were decoupled, this would appear to benefit females, as they could display high hunting aggression as juveniles, without killing potential mates before copulation as an adult. Thus, one would expect strong selection for individuals that can dissociate aggression between hunting and mating contexts. However, there is evidence for a lack of lability in aggression in several species. In the fishing spider Dolomedes triton, female aggression towards prey is positively correlated with aggression towards conspecific males (Arnqvist and Henriksson 1997; Johnson 2001). Positive correlations between female aggression towards prey and the probability of attacking a potential mate have also been demonstrated in funnel-web spiders (Riechert and Hedrick 1993), cob-web spiders (Pruitt et al. 2008) and a wolf spider (Rabaneda-Bueno et al. 2014). There is also evidence for positive correlations between juvenile and adult aggression towards prey in sexually cannibalistic species, which further supports the notion that aggression in sexually cannibalistic species is linked across contexts (Johnson and Sih 2005).
However, if aggressive spillover is a major factor in maintaining sexual cannibalism in nature, then there are two relatively untested predictions. Firstly, if aggression against prey and cannibalistic aggression are correlated within a species, it is likely they are influenced by similar neural and/or genetic mechanisms. It is therefore possible that when sexual cannibalism reduces female fitness, and so is selected against, it may also result in a decline in overall aggression towards prey. In other words, species in which females cannot afford cannibalism may be constrained to lower aggression than cannibalistic species. Secondly, within cannibalistic species, the evolution of male aggression towards prey may also be constrained if it is correlated with potentially costly behaviours. For example, aggression is known to be correlated with boldness in other cannibalistic species (Johnson and Sih 2005; Johnson and Sih 2007). Although males of some cannibalistic species are known to benefit from aggression towards prey and high juvenile feeding rates (Barry 2013), they may increase their risk of being cannibalised if they are bold in the presence of females (Lelito and Brown 2006). This could constrain the evolution of high aggression in males of species where females are cannibalistic.
Determining whether or not sexual cannibalism is correlated with aggression towards prey is important for understanding why the behaviour has evolved and is maintained in nature, it is also because correlations between behaviours can reduce behavioural plasticity (Sih et al. 2012), the presence such correlations in a cannibalistic species could have important ecological implications (Moran et al. 1996; Fisher et al. 2018; Lichtenstein et al. 2019). We examined aggression across life-stages and context in two species of praying mantis residing in New Zealand: the highly sexually cannibalistic Miomantis caffra and the less cannibalistic Orthodera novaezealandiae (Fea et al. 2013; Walker and Holwell 2015). We tested the following predictions: (1) female M. caffra will on average be more aggressive towards prey than female O. novaezealandiae as juveniles and adults, (2) female M. caffra will on average be more aggressive towards prey than male M. caffra as juveniles and adults, (3) aggression towards prey will be correlated between lifestages in M. caffra, and (4) adult aggression in female M. caffra will be positively correlated with the likelihood of attacking potential mates.
Methods
Study species
Two species of praying mantis were used in this study, O. novaezealandiae and M. caffra. O. novaezealandiae is the only praying mantis species known to be native to New Zealand; it is most commonly found in an open habitat and shrubland (Ramsay 1990) and rarely cannibalises (Fea et al. 2013). O. novaezealandiae were collected from grassland and woodland areas around Auckland, NZ. The second species, M. caffra, is an invasive South African mantis that is estimated to have been introduced to New Zealand’s north island in 1978. Female M. caffra have been shown to cannibalise males in 60% of encounters (Walker and Holwell 2015). Moreover, sexual cannibalism in M. caffra is always pre-copulatory (Fea et al. 2013; Walker and Holwell 2015). M. caffra is now well-established in many areas of New Zealand’s north island and is commonly found in suburban gardens. We collected M. caffra from the suburbs surrounding Auckland. Both species were collected from February to March 2017 and February 2019. To ensure that all individuals were virgin, only juveniles were collected. All mantids were housed individually in inverted 750-ml plastic cups with a mesh ceiling, misted daily and fed houseflies (Musca domestica) ad libitum.
Aggression towards prey
Mantids were starved for 2 days prior to testing to standardise hunger levels and were weighed immediately before testing. Note that because insects are not known to vary significantly in size within instars (Chown and Gaston 2010), weight can be compared within instars irrespective of age. The mantids were then placed in a six-sided plastic container measuring 70 × 120 × 80 mm (H × W × D). When measuring predatory responses, using a virtual prey item can be useful for standardising the variation in activity that would be present in live prey (Ioannou et al. 2012). We created a moving virtual prey item (see supplementary material) in Adobe Macromedia Flash 8. The virtual prey item was displayed on a digital screen affixed to the side of the mantis’ enclosure. Similar methods have been used previously to induce predatory responses in praying mantids and jumping spiders (Yamawaki 2003; Bartos and Minias 2016). To quantify aggression, we recorded whether or not the mantis attacked the virtual prey using its raptorial forelimbs. The mantis was given a maximum time of 5 min to attack the virtual prey. The mantis was then offered a single housefly and given a maximum time of 10 min to capture the fly. If the mantis consumed the fly, we would once again test the mantis’ aggression towards the virtual prey. This process was repeated until (1) the mantis stopped eating the offered flies (we assumed that mantids that would not attack real prey would not attack virtual prey) or (2) the maximum number of repeats (five) was reached. Repeating the process allowed us to compare aggression in response to changes in prior food consumption. This method was used for both M. caffra (nmale = 16, nfemale = 16) and O. novaezealandiae (nmale = 16, nfemale = 14) at both the sub-adult (penultimate instar) and adult stages, such that a maximum of 10 repeats were conducted for each individual.
Aggression towards potential mates
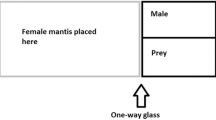
To determine the likelihood that a female would cannibalise a male, a randomly chosen conspecific adult male was introduced to the opposite side of a mesh enclosure measuring 450 × 450 × 450 mm (H × W × D) containing an adult female. Once again, to standardise hunger females were starved for 2 days prior to testing. Mating trials (n = 12 for M. caffra and n = 7 for O. novaezealandiae) took place at night as there is evidence to suggest that male mantids are more likely to approach females after dusk (Fea et al. 2013; Walker and Holwell 2015). Males and females were paired between 19:00 and 20:00 and left for 12 h. After 12 h, we recorded whether or not the male had been devoured. We assumed that the consumption of males only took place in a mating context as female mantids are typically sit-and-wait predators (Hurd 1999; Barry 2013) and adult males are only known to approach females for the purposes of mating (Gemeno and Claramunt 2006; Lelito and Brown 2006). Also, because mantids are often more active upon being moved to a new enclosure (personal observations of AF), individuals were monitored for several minutes after being introduced to the mating enclosures. This allowed us to discard any instances of cannibalism that may have occurred during the initial acclimatisation period. Individuals were used only once in mating trials.
Analyses
We used a generalized linear mixed-effects model with binomial error correction to determine whether the probability that an individual would attack the virtual prey item varied in response to age, sex or species. The model fixed effects consisted of a three-way interaction between age (sub-adult or adult), sex and species. A random effect for individual was included where the intercept remained constant at one. To determine whether certain groups became less aggressive due to satiating sooner, we constructed a second model in which we added the number of flies an individual had eaten immediately prior to exposure to the virtual prey item as a fixed effect; this created a four-way interaction with sex, age or species. Once again, individual was included in the model as a random effect in which the intercept remained constant at one. We used a separate binomial GLM to test for a relationship between aggression towards prey and the probability that a female would cannibalise a male. Here, females and males were only used once; thus, no random effects were needed. To determine the effect of age, sex and species on individual weight, we used a generalised linear mixed-effects model with Gaussian error where age, sex and species formed a three-way interaction and individual was included as a random effect with a constant intercept of one.
We also used Spearman’s rank correlation to see if there was a relationship between juvenile weight and juvenile attack frequency. Note that we did not attempt to correlate aggression with adult weight, as adult weight was more likely to have been a product of the standardised laboratory feeding regime as opposed to natural feeding rates. Spearman’s rank correlations were also used to check for a relationship between juvenile attack probability and adult attack probability. Behavioural repeatability describes the proportion of behavioural variation in the population that can be explained by variation at the individual level such that \( R=\frac{V_G}{\left({V}_G+{V}_I\right)} \), where VG is the amount of variation expressed across the population and VI is the average variation within an individual expressed across repeated trait measures. We calculated repeatability for males and females in both species using the rptR package in R (Schielzeth and Nakagawa 2011), where attack on the virtual prey item (binomial) was the response variable and individual/organism ID was a random effect. Repeatability estimates were adjusted to account for age and repeat which are potential confounding factors. The model was run for 1000 bootstrap repeats. All statistics were carried out in R version 3.3.0 (R Core Team 2016) and GLMMs were conducted using the lme4 package (Bates et al. 2015). Because our model for organism weight was non-binary, we used the lmerTest package to generate p values (Kuznetsova et al. 2017).
Results
Miomantis caffra aggression
There was a significant interaction between sex and life stage on overall attack frequency on the virtual prey for M. caffra (Fig. 1a; df = 56, z = 3.874, p < 0.001), meaning that the effect of age on aggression was different for male and female M. caffra. Sub-adult females had a higher attack frequency than sub-adult males, but this difference was non-significant (df = 56, z = 1.338, p = 0.181). However, adult attack frequency was significantly higher in females than in males (df = 56, z = 4.872, p < 0.001). Female attack frequency did not change significantly between the sub-adult and adult stage (df = 56, z = 0.556, p = 0.578), but adult males were significantly less likely to attack than sub-adult males (df = 56, z = −4.633, p < 0.001). Over the 12 cannibalism trials with M. caffra, five males were cannibalised. The frequency at which an adult female attacked the virtual prey was not significantly associated with whether or not the female would cannibalise a male (df = 11, z = 1.518, p = 0.129).
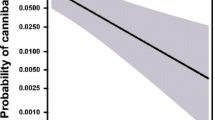
In sub-adult males and females, the probability of an individual attacking the virtual prey item was not significantly affected by the number of flies that had been consumed (Fig. 2a; males: df = 26, z = 0.171, p = 0.864; females: df = 26, z = 1.091, p = 0.275). There were no significant interaction between the number of flies consumed and sex (df = 26 z = 0.682, p = 0.495). In adults, the effect of the number of flies consumed on attack probability was significantly different for adult males and females (Fig. 2b; df = 26, z = 2.704, p = 0.00685). Attack probability declined significantly in adult males in response to the number of flies they had been eaten prior to being exposed to the virtual prey, whereas attack probability in adult females showed a marginally non-significant positive response to fly consumption (Fig. 2b; males: df = 26, z = − 2.018, p = 0.0436; females: df = 26, z = 1.833, p = 0.0668).
Orthodera novaezealandiae aggression
There was a significant interaction between sex and life stage on attack frequency towards the virtual prey item in O. novaezealandiae (Fig. 1b; df = 56, z = 2.222, p = 0.026), meaning that the effect of age on aggression was different for males and females. Attack frequency was not significantly different between sub-adult males and females (Fig. 1b; df = 56, z = 0.031, p = 0.975). Adult female O. novaezealandiae had a marginally non-significant higher attack frequency than adult males (df = 56, z = 1.795, p = 0.072). Female attack frequency did not change significantly between the sub-adult and adult stage (df = 56, z = 0.951, p = 0.342). There was a marginal but non-significant trend for attack frequency to be higher in sub-adult than adult males (df = 56 z = −1.732, p = 0.083). Cannibalism was only observed once out of seven mating trials in O. novaezealandiae.
In sub-adult males and females, the probability that an individual would attack the virtual prey item was not significantly affected by the number of flies consumed (males: df = 23, z = −1.496, p = 0.135; females: df = 23, z = − 0.223, p = 0.823), and the interaction between sex and the number of flies consumed was also non-significant (df = 23, z = 0.817, p = 0.414). Similarly, the probability of individuals attacking the virtual prey item was not significantly affected by the number of flies consumed in adult females (df = 23, z = − 0.617, p = 0.537), but the probability of adult males attacking the virtual prey item decreased significantly as the number of flies consumed increased (df = 23, z = − 2.041, p = 0.0413). There was no significant interaction between the number of flies consumed and sex in adults (df = 23, z = 1.086, p = 0.277).
Comparison of aggression of both species
Attack rate in female M. caffra was significantly higher than that of O. novaezealandiae females at both the sub-adult and adult life stage (sub-adult: df = 56, z = 2.58, p = 0.01; adult: df = 56, z = 2.301, p = 0.021). Attack rates in male M. caffra did not differ significantly from that of O. novaezealandiae males in sub-adults or adults (sub-adult: df = 56, z = 1.452, p = 0.146; adult: df = 56, z = − 0.882, p = 0.377). The three-way interaction between species, life stage and aggression was not significant (df = 56, z = 1.395, p = 0.163), meaning that the difference in the effect of age on aggression in males and females was not different between M. caffra and O. novaezealandiae.
Repeatability
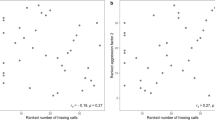
For male and female M. caffra, we found no significant correlation between the number of times an individual attacked the virtual prey item as juveniles and adults (Fig. 3a; Spearman’s rank: males: S = 543.56, rho = 0.20, p = 0.46; females: S = 811.67, rho = − 0.19, p = 0.47). In O. novaezealandiae juvenile and adult attack rates were correlated in females but not males (Fig. 3b; Spearman’s rank: males: S = 519.95, rho = 0.235, p = 0.38; females: S = 86.795, rho = 0.762, p = 0.0025). In concordance with the findings of Bell et al. (2009) for invertebrates, female aggression was more repeatable than male aggression for both M. caffra and O. novaezealandiae (Table 1). However, only aggression in female O. novaezealandiae was more repeatable than the average repeatability (0.32) calculated from a range of studies on other organisms (Bell et al. 2009). Thus, we found evidence for consistency in overall aggression and a correlation between juvenile and adult aggression in female O. novaezealandiae, but not male O. novaezealandiae or either sex in M. caffra. Frequency histograms for the repeatability estimates can be found in the supplementary material.
Body mass
Body mass was not significantly different between male and female sub-adult M. caffra (Fig. 4; df = 98, t = 1.235, p = 0.221). However, adult female M. caffra were significantly heavier than adult males (df = 98, t = 10.333, p < 0.001) and sub-adult females (df = 98, t = 17.207, p < 0.001). Adult male M. caffra were significantly heavier than sub-adult males (df = 98, t = 3.227, p = 0.00226). For O. novaezealandiae, there was no significant difference between the sub-adult weights of males or females (df = 98, t = 0.569, p = 0.571). The difference in weight between adult and sub-adult male O. novaezealandiae was marginally non-significant (df = 98, t = 1.920, p = 0.0606). Adult female O. novaezealandiae were significantly heavier than adult males (df = 98, t = − 4.522, p < 0.001) and sub-adult females (df = 98, t = − 7.005, p < 0.001). We also found a significant correlation between the number of times a juvenile attacked the virtual prey item and juvenile weight in O. novaezealandiae but not M. caffra (Fig. 5; Spearman’s rank: O. novaezealandiae: S = 581.71, rho = 0.563, p = 0.00981; M. caffra: S = 5952.4, rho = − 0.091, p = 0.6204).
Discussion
Behavioural correlations and behavioural repeatability have previously been used to explain the occurrence of pre-copulatory cannibalism by suggesting that cannibalism is a by-product of strong selection for aggression towards non-conspecific prey. As a result, aggression in individuals from species that display high rates of pre-copulatory cannibalism should be consistent across time and environmental context. In this study, although adult female M. caffra were significantly more aggressive towards prey and more cannibalistic than adult female O. novaezealandiae, we only found evidence for behavioural repeatability in O. novaezealandiae. This finding directly contradicts the aggressive spillover theory for pre-copulatory cannibalism. We have also shown that male M. caffra can dramatically reduce their levels of aggression towards prey upon eclosing as adults, showing that aggression can vary independently across instars in a species where females frequently devour males prior to copulation. Moreover, we found no association between adult aggression and pre-copulatory cannibalism in M. caffra, providing further evidence that pre-copulatory cannibalism need not be a by-product of aggression towards prey. Our results suggest that aggressive spillover may not explain high levels of pre-copulatory cannibalism, even in clades where sexual cannibalism is common. It may be that the high levels of pre-copulatory cannibalism seen in some species are not a behavioural by-product, but instead have been selected for because of the direct benefits cannibalism brings to females.
Previous studies show that consistency in aggression throughout ontogeny may be linked to the presence of sexual cannibalism (Arnqvist and Henriksson 1997; Johnson and Sih 2005; Johnson and Sih 2007). We found no correlation between juvenile aggression and adult aggression in M. caffra and very little evidence for behavioural repeatability, showing that consistency in aggression across time is not linked to the high frequency of sexual cannibalism in M. caffra. Previous studies have demonstrated that the individual and population-level benefits of sexual cannibalism may be highly dependent on the environment (Buskirk et al. 1984; Fisher et al. 2018). For example, sexual cannibalism may increase the incidence of virgin deaths if females continue to cannibalise males when encounters are rare, such as in sparse populations. It would therefore be advantageous for females to be able to vary how frequently they cannibalise males in response to environmental cues, such as mate availability. Previous work suggests that the presence of a behavioural correlation lowers the plastic potential of individual behaviour (Sih et al. 2012); thus, emergence of correlated behaviours could be strongly selected against in sexually cannibalistic species to allow for greater variation in cannibalism rates.
Conversely, we found evidence for behavioural repeatability in adult female O. novaezealandiae. We have also shown a positive correlation between how often individuals attacked the virtual prey item and weight in O. novaezealandiae. This suggests a link between repeatable high aggression and mass relative to conspecifics. Thus, behavioural consistency may be selected for to increase individual competitiveness through traits such as body size, which is likely dependent on the long-term feeding behaviour of an individual (Jones and DiRienzo 2018). However, there may also be a trade-off between the positive and negative fitness effects of behavioural repeatability. For example, if repeatability in aggression causes highly aggressive individuals to be aggressive towards potential mates, and aggression towards potential mates has negative fitness consequences, then high levels of aggression may be selected against in such species. Fitness trade-offs associated with behavioural correlations may provide some explanation as to why female O. novaezealandiae are less aggressive than female M. caffra across all the contexts we tested.
Juvenile prey attack frequency was very similar for males and females of both species, implying that both sexes rely on high feeding rates during development to maximise their lifetime fitness and the probability of reaching adulthood. In praying mantids, males are typically the dispersing sex and therefore require functioning flight muscles and wings to locate potential mates (Maxwell et al. 2010). The development of wings and associated musculature is known to be energetically costly in other insects (Mole and Zera 1993; Langellotto et al. 2000); thus, male mantids may feed voraciously as juveniles to accommodate the energetic demands of adult dispersal. Evidence for the effect of juvenile feeding on adult dispersal is provided by the false garden mantis (Pseudomantis albofimbriata), where males kept on a low feeding regime as juveniles were slower at finding a mate than those that were well fed (Barry 2013). For female mantids, adult size is likely a determinant of maximum egg production (Allen et al. 2014). In fishing spiders of the genus Dolomedes (family: Pisauridae) fixed female size (size independent of current feeding rate, i.e. cephalothorax width) was shown to significantly increase reproductive output (Spence et al. 1996; Arnqvist and Henriksson 1997; Johnson 2001), and was more important for determining female fecundity than adult feeding rate (Johnson 2001). Hence, it could be that the selection pressure for high juvenile aggression is similar for males and females because both sexes are equally reliant on nutrient intake to maximise adult fitness. This may be particularly true in species where males are required to develop energetically costly flight traits to allow for long distance dispersal.
Although aggression was similar for juvenile male and female M. caffra, adult females were significantly more aggressive than adult males. The difference in adult male and adult female aggression was due to males becoming less aggressive upon reaching adulthood, and satiating sooner. Although it is not certain why this occurs, one possibility is that males feed less as adults to limit their weight thereby allowing for easier flight dispersal. Lowering body mass increases the flight muscle to body mass ratio in flying insects and has been shown to enhance male fitness in dragonflies (Marden 1989). Female M. caffra maintained their high aggression into adulthood and did not get less aggressive in response to feeding. This implies that high aggression towards prey is similarly advantageous for juvenile and adult female M. caffra. Because males are typically the dispersing sex in mantids, and female M. caffra are flightless, low body mass would not provide a fitness advantage to females. Instead, maintaining a high feeding rate may raise female fitness via increased egg production (Murphy et al. 1983; Barry et al. 2008).
In this study, high levels of adult aggression towards prey in M. caffra did not pre-dispose females to pre-copulatory cannibalism, implying that sexual cannibalism is not a behavioural by-product. If sexual cannibalism is not a by-product of aggression towards prey, then pre-copulatory sexual cannibalism must sometimes occur in nature because it is adaptive in itself. This could be true if males are highly nutritious for females, as has been shown in the funnel spider Agelenopsis pennsylvanica and the fishing spider Dolomedes tenobrosus, where females that had cannibalised a male prior to copulation showed a greater number of offspring emergence (Berning et al. 2012; Schwartz et al. 2016). Similarly, in populations where males vary greatly in terms of their quality, then pre-copulatory cannibalism may be selected for as a method of mate choice (Prenter et al. 2006; Kralj-Fišer et al. 2012). In the wolf spider Schizocosa ocreata (family: Lycosidae), females are known to cannibalise smaller males over larger males (Persons and Uetz 2005). Hence, pre-copulatory cannibalism can be selected for directly and does not only occur as a by-product of selection for aggression towards prey.
Conclusions
The aggressive spillover theory for pre-copulatory cannibalism proposes that aggression in some species is linked across developmental and ecological contexts and that sexual cannibalism is a by-product of this aggression. Our study has shown that high rates of pre-copulatory cannibalism may not be an indication that aggression is correlated across contexts among individuals. Instead, sexual cannibalism may only be able to persist in some species if cannibalism rates are determined by environmental cues, not other behaviours. Secondly, we have shown that the presence of consistent levels of aggression across life stages may lead to overall lower levels of aggression in some species. This may be due to the fitness benefits of high levels of aggression being outweighed by the potential costs if aggression towards prey is correlated with aggression towards potential mates. Finally, we show that males of the sexually cannibalistic species of mantis M. caffra can become less aggressive upon reaching adulthood, suggesting that this species can vary aggression depending on age. Our evidence supports the idea that aggression can evolve in a way that allows for variation across contexts within individuals of sexually cannibalistic species; but also that lower levels of aggression may be selected for if aggression is inherently correlated across contexts in a particular species. Future work investigating the potential for sexual cannibalism rates to be plastic in response to environmental cues could be useful for conservation.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Allen LE, Barry KL, Holwell GI (2014) Different paths to sexual size dimorphism in two praying mantids, Pseudomantis albofimbriata and Hierodula majuscula. Insect Sci 21:227–233
Arnqvist G (1992) Courtship behavior and sexual cannibalism in the semi-aquatic fishing spider, Dolomedes fimbriatus (Clerck) (Araneae: Pisauridae). J Arachnol 20:222–226
Arnqvist G, Henriksson S (1997) Sexual cannibalism in the fishing spider and a model for the evolution of sexual cannibalism based on genetic constraints. Evol Ecol 11:255–273
Barry KL (2013) You are what you eat: food limitation affects reproductive fitness in a sexually cannibalistic praying mantid. PLoS One 8:e78164
Barry KL, Holwell GI, Herberstein ME (2008) Female praying mantids use sexual cannibalism as a foraging strategy to increase fecundity. Behav Ecol 19:710–715
Barry KL, Holwell GI, Herberstein ME (2009) Male mating behaviour reduces the risk of sexual cannibalism in an Australian praying mantid. J Ethol 27:377–383
Bartos M, Minias P (2016) Visual cues used in directing predatory strikes by the jumping spider Yllenus arenarius (Araneae, Salticidae). Anim Behav 120:51–59
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783
Berning AW, Gadd RD, Sweeney K, MacDonald L, Eng RY, Hess ZL, Pruitt JN (2012) Sexual cannibalism is associated with female behavioural type, hunger state and increased hatching success. Anim Behav 84:715–721
Buskirk RE, Frohlich C, Ross KG (1984) The natural selection of sexual cannibalism. Am Nat 123:612–625
Chown SL, Gaston KJ (2010) Body size variation in insects: a macroecological perspective. Biol Rev 85:139–169
Cote J, Clobert J, Brodin T, Fogarty S, Sih A (2010) Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos Trans R Soc Lond B Biol Sci 365:4065–4076
Dingemanse NJ, Both C, Van Noordwijk AJ, Rutten AL, Drent PJ (2003) Natal dispersal and personalities in great tits (Parus major). Proc Biol Sci 270:741–747
Dingemanse NJ, Wright J, Kazem AJ, Thomas DK, Hickling R, Dawnay N (2007) Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138
Elgar MA (1992) Sexual cannibalism in spiders and other invertebrates. Cannibalism: ecology and evolution among diverse taxa, 1st edn. Oxford University Press, Oxford, pp 128–155
Elgar MA, Nash DR (1988) Sexual cannibalism in the garden spider Araneus diadematus. Anim Behav 36:1511–1517
Fea MP, Stanley MC, Holwell GI (2013) Fatal attraction: sexually cannibalistic invaders attract naive native mantids. Biol Lett 9:20130746
Fisher AM, Cornell SJ, Holwell GI, Price TAR (2018) Sexual cannibalism and population viability. Ecol Evol 8:6630–6670
Gemeno C, Claramunt J (2006) Sexual approach in the praying mantid Mantis religiosa (L.). J Insect Behav 19:731–740
Hebets EA (2003) Subadult experience influences adult mate choice in an arthropod: exposed female wolf spiders prefer males of a familiar phenotype. Proc Natl Acad Sci U S A 100:13390–13395
Huntingford FA (1976) The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim Behav 14:245–260
Hurd LE (1999) Ecology of praying mantids. In: Prete FR, Wells H, Wells PH, Hurd LE (eds) The praying mantids, 1st edn. The John Hopkins University Press, Maryland, pp 43–60
Hurd LE, Eisenberg RM, Fagan WF, Tilmon KJ, Snyder WE, Vandersall KS, Datz SG, Welch JD (1994) Cannibalism reverses male-biased sex ratio in adult mantids: female strategy against food limitation? Oikos 69:193–198
Ioannou CC, Guttal V, Couzin ID (2012) Predatory fish select for coordinated collective motion in virtual prey. Science 337:1212–1215
Johnson JC (2001) Sexual cannibalism in fishing spiders (Dolomedes triton): an evaluation of two explanations for female aggression towards potential mates. Anim Behav 61:905–914
Johnson JC, Sih A (2005) Precopulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioral syndromes. Behav Ecol Sociobiol 58:390–396
Johnson JC, Sih A (2007) Fear, food, sex and parental care: a syndrome of boldness in the fishing spider, Dolomedes triton. Anim Behav 74:1131–1138
Jones C, DiRienzo N (2018) Behavioral variation post-invasion: resemblance in some, but not all, behavioral patterns among invasive and native praying mantids. Behav Process 153:92–99
Kralj-Fišer S, Schneider JM, Justinek Ž, Kalin S, Gregorič M, Pekár S, Kuntner M (2012) Mate quality, not aggressive spillover, explains sexual cannibalism in a size-dimorphic spider. Behav Ecol Sociobiol 66:145–151
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1548–7660
Langellotto GA, Denno RF, Ott JR (2000) A trade-off between flight capability and reproduction in males of a wing-dimorphic insect. Ecology 81:865–875
Lelito JP, Brown WD (2006) Complicity or conflict over sexual cannibalism? Male risk taking in the praying mantis Tenodera aridifolia sinensis. Am Nat 168:263–269
Lichtenstein JL, Daniel KA, Wong JB, Wright CM, Doering GN, Costa-Pereira R, Pruitt JN (2019) Habitat structure changes the relationships between predator behavior, prey behavior, and prey survival rates. Oecologia 190:297–308
Marden JH (1989) Bodybuilding dragonflies: costs and benefits of maximizing flight muscle. Physiol Zool 62:505–521
Maxwell MR, Barry KL, Johns PM (2010) Examinations of female pheromone use in two praying mantids, Stagmomantis limbata and Tenodera aridifolia sinensis (Mantodea: Mantidae). Ann Entomol Soc Am 103:120–127
Mole S, Zera AJ (1993) Differential allocation of resources underlies the dispersal-reproduction trade-off in the wing-dimorphic cricket, Gryllus rubens. Oecologia 93:121–127
Moran MD, Rooney TP, Hurd LE (1996) Top-down cascade from a bitrophic predator in an old-field community. Ecology 77:2219–2227
Murphy DD, Launer AE, Ehrlich PR (1983) The role of adult feeding in egg production and population dynamics of the checkerspot butterfly Euphydryas editha. Oecologia 56:257–263
Persons MH, Uetz GW (2005) Sexual cannibalism and mate choice decisions in wolf spiders: influence of male size and secondary sexual characters. Anim Behav 69:83–94
Pruitt JN, Riechert SE (2012) The ecological consequences of temperament in spiders. Curr Zool 58(4):589–596
Prenter J, MacNeil C, Elwood RW (2006) Sexual cannibalism and mate choice. Anim Behav 71:481–490
Pruitt JN, Riechert SE, Jones TC (2008) Behavioural syndromes and their fitness consequences in a socially polymorphic spider, Anelosimus studiosus. Anim Behav 76:871–879
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. https://www.R-project.org/
Rabaneda-Bueno R, Aguado S, Fernández-Montraveta C, Moya-Laraño J (2014) Does female personality determine mate choice through sexual cannibalism? Ethology 120:238–248
Ramsay GW (1990) Mantodea (Insecta), with a review of aspects of functional morphology and biology. Fauna N Z 19:1–102
Riechert SE, Hedrick AV (1993) A test for correlations among fitness-linked behavioural traits in the spider Agelenopsis aperta (Araneae, Agelenidae). Anim Behav 46:669–675
Roggenbuck H, Pekár S, Schneider JM (2011) Sexual cannibalism in the European garden spider Araneus diadematus: the roles of female hunger and mate size dimorphism. Anim Behav 81:749–755
Schielzeth H, Nakagawa S (2011) rptR: repeatability for Gaussian and non-Gaussian data. R package
Schwartz SK, Wagner WE Jr, Hebets EA (2016) Males can benefit from sexual cannibalism facilitated by self-sacrifice. Curr Biol 26:2794–2799
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sih A, Cote J, Evans M, Fogarty S, Pruitt J (2012) Ecological implications of behavioural syndromes. Ecol Lett 15:278–289
Smith BR, Blumstein DT (2008) Fitness consequences of personality: a meta-analysis. Behav Ecol 19:448–455
Spence JR, Zimmermann M, Wojcicki JP (1996) Effects of food limitation and sexual cannibalism on reproductive output of the nursery web spider Dolomedes triton (Araneae: Pisauridae). Oikos 75:373–382
Walker LA, Holwell GI (2015) Sexual cannibalism in a facultative parthenogen: the springbok mantis (Miomantis caffra). Behav Ecol 27:851–856
Wilder SM, Rypstra AL (2008) Sexual size dimorphism predicts the frequency of sexual cannibalism within and among species of spiders. Am Nat 172:431–440
Yamawaki Y (2003) Responses to worm-like-wriggling models by the praying mantis: effects of amount of motion on prey recognition. J Ethol 21:123–129
Acknowledgements
The authors would like to thank Dr. Samantha C. Patrick for her help with the analysis and the staff and students at the School of Biological Sciences, University of Auckland who assisted in collecting the mantids.
Funding
This work was funded by the Natural Environment Research Council: Adapting to the Challenges of a Changing Environment Doctoral Training Partnership studentship to AF and a Natural Environment Research Council grant to TP (NE/P002692/1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by J. Pruitt
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 306 kb).
(MP4 8229 kb).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fisher, A.M., Holwell, G.I. & Price, T.A.R. Behavioural correlations and aggression in praying mantids. Behav Ecol Sociobiol 74, 61 (2020). https://doi.org/10.1007/s00265-020-02839-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02839-8