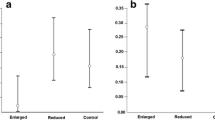
Abstract
Female mass in most altricial birds reaches its maximum during breeding at egg laying, which coincides temporally with the fertile phase when extra-pair paternity (EPP) is determined. Higher mass at laying may have two different effects on EPP intensity. On the one hand, it would lead to increased wing loading (body mass/wing area), which may impair flight efficiency and thereby reduce female’s capacity to resist unwanted extra-pair male approaches (sexual conflict hypothesis). On the other hand, it would enhance female condition, favouring her capacity to evade mate guarding and to search for extra-pair mates (female choice hypothesis). In both cases, higher female mass at laying may lead to enhanced EPP. To test this prediction, we reduced nest building effort by adding a completely constructed nest in an experimental group of female pied flycatchers (Ficedula hypoleuca). Our treatment caused an increase in mass and thereby wing loading and this was translated into a significantly higher EPP in the manipulated group compared with the control group as expected. There was also a significant negative relationship between EPP and laying date and the extent of the white wing patch, an index of female dominance. More body reserves at laying mean not only a higher potential fecundity but a higher level of EPP as well. This interaction had not previously received due attention but should be considered in future studies of avian breeding strategies.
Significance statement
While most research has been focused on determining possible criteria for extra-pair mate choice by females, less effort has been made on establishing if female traits are related to EPP and its intensity. One such trait is mass at laying which attains its highest level for breeding females of altricial birds. Our study indicates that a higher mass during the fertile phase not only has implications for female fecundity and predation risk but also for EPP in the resulting brood as more mass means a higher EPP.

Similar content being viewed by others
References
Alatalo RV, Gottlander K, Lundberg A (1987) Extra-pair copulations and mate guarding in the polyterritorial pied flycatcher, Ficedula hypoleuca. Behaviour 101:139–155
Arnqvist G, Kirkpatrick M (2005) The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am Nat 165:S26–S37
Bailey IE, Morgan KV, Oschadleus HD, DeRuiter SL, Meddle SL, Healy SD (2016) Nest-building males trade off material collection costs with territory value. Emu 116:1–8
Björklund M, Westman B (1983) Extra-pair copulations in the pied flycatcher (Ficedula hypoleuca). Behav Ecol Sociobiol 13:271–275
Blomqvist D, Andersson M, Küpper C, Cuthill IC, Kis J, Lanctot RB, Sandercock BK, Székely T, Wallander J, Kempenaers B (2002) Genetic similarity between mates and extra-pair parentage in three species of shorebirds. Nature 419:613–615
Boulton RA, Zuk M, Shuker DM (2018) An inconvenient truth: the unconsidered benefits of convenience polyandry. Trends Ecol Evol 33:904–915
Bouwman KM, Komdeur J (2005) Old female reed buntings (Emberiza schoeniclus) increase extra-pair paternity in their broods when mated to young males. Behaviour 142:1449–1463
Canal D, Potti J, Dávila JA (2011) Male phenotype predicts extra pair paternity in pied flycatchers. Behaviour 148:691–712
Canal D, Jovani R, Potti J (2012) Male decisions or female accessibility? Spatiotemporal patterns of extra pair paternity in a songbird. Behav Ecol 23:1146–1153
Cantarero A, Laaksonen T, Järvistö PE, Gil D, López-Arrabé J, Redondo AJ, Moreno J (2015a) Nest defense behaviour and testosterone levels in female pied flycatchers. Ethology 121:946–957
Cantarero A, Laaksonen T, Järvistö PE, López-Arrabé J, Gil D, Moreno J (2016a) Testosterone levels in relation to size and UV reflectance of achromatic plumage traits of female pied flycatchers. J Avian Biol 48:243–254
Cantarero A, López-Arrabé J, Moreno J (2015b) Selection of nest-site and nesting material in the eurasian nuthatch Sitta europaea. Ardea 103:91–94
Cantarero A, López-Arrabé J, Palma A, Redondo AJ, Moreno J (2014) Males respond to female begging signals of need: a handicapping experiment in the pied flycatcher Ficedula hypoleuca. Anim Behav 94:167–173
Cantarero A, López-Arrabé J, Plaza M, Saavedra-Garcés I, Moreno J (2016b) Males feed their mates more and take more risks for nestlings with larger female-built nests: an experimental study in the nuthatch Sitta europaea. Behav Ecol Sociobiol 70:1141–1150
Curio E (1959) Beitrage zur populations okologie des trauerschnappers (Ficedula h. hypoleuca Pallas). Zool Jahrb (Systematik) 87:185–230
Chek AA, Lifjeld JT, Robertson RJ (1993) Captive study of copulation in the pied flycatcher Ficedula hypoleuca. Fauna Norvegica Ser C Cinclus 16:67–73
Deeming C (2013) Feathering the nest. Biologist 60:26–30
Ellegren H, Lifjeld JT, Slagsvold T, Primmer CR (1995) Handicapped males and extrapair paternity in pied flycatchers: a study using microsatellite markers. Mol Ecol 4:739–744
Forstmeier W, Martin K, Bolund E, Schielzeth H, Kempenaers B (2011) Female extrapair mating behavior can evolve via indirect selection on males. P Natl Acad Sci USA 108:10608–10613
Forstmeier W, Nakagawa S, Griffith SC, Kempenaers B (2014) Female extra-pair mating: adaptation or genetic constraint? Trends Ecol Evol 29:456–464
Garamszegi LZ, Møller AP (2004) Extrapair paternity and the evolution of bird song. Behav Ecol 15:508–519
Gelter HP, Tegelström H (1992) High frequency of extra-pair paternity in Swedish pied flycatchers revealed by allozyme electrophoresis and DNA fingerprinting. Behav Ecol Sociobiol 31:1–7
Gill SA, Stutchbury BJM (2005) Nest building is an indicator of parental quality in the monogamous neotropical buff-breasted wren (Thryothorus leucotis). Auk 122:1169–1181
Griffith SC, Blomqvist D, Andersson M et al (2003) Why do birds engage in extra-pair copulation? Nature 422:833
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Griggio M, Matessi G, Pilastro A (2003) Male rock sparrow (Petronia petronia) nest defence correlates with female ornament size. Ethology 109:659–669
Griggio M, Valera F, Casas A, Pilastro A (2005) Males prefer ornamented females: a field experiment of male choice in the rock sparrow. Anim Behav 69:1243–1250
Hansell M (2000) Bird nests and construction behaviour. Cambridge University Press, Cambridge
Kalinowski S, Taper M, Marshall T (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1009–1106
Kempenaers B, Verheyen GR, den Broeck MV, Burke T, Broeckhoven CV, Dhondt A (1992) Extra-pair paternity results from female preference for high-quality males in the blue tit. Nature 357:494–496
Kullberg C, Metcalfe NB, Houston DC (2002) Impaired flight ability during incubation in the pied flycatcher. J Avian Biol 33:179–183
Lambrechts M, Adriaensen F, Ardia DR et al (2010) The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol 45:1–26
Leder EH, Karaiskou N, Primmer CR (2008) Seventy new microsatellites for the pied flycatcher, Ficedula hypoleuca and amplification in other passerine birds. Mol Ecol Resour 8:874–880
Lifjeld JT, Slagsvold T, Dale S, Ellegren H (1997a) A sexually selected paradox in the pied flycatcher: attractive males are cuckolded. Auk 114:112–115
Lifjeld JT, Slagsvold T, Ellegren H (1997b) Experimental mate switching in pied flycatchers: male copulatory access and fertilization success. Anim Behav 53:1225–1232
Lothery CJ, Thompson CF, Lawler ML, Sakaluk SK (2014) Food supplementation fails to reveal a trade-off between incubation and self-maintenance in female house wrens. PLoS One 9:e106260
Lundberg A, Alatalo RV (1992) The pied flycatcher. Poyser, London
Martínez-de la Puente J, Merino S, Lobato E, Moreno J, Tomás G, Morales J (2009) Male nest-building activity influences clutch mass in pied flycatchers Ficedula hypoleuca. Bird Stud 56:264–267
Mennerat A, Charmantier A, Jørgensen C, Eliassen S (2018) Correlates of complete brood failure in blue tits: could extra-pair mating provide unexplored benefits to females? J Avian Biol 49:e01701
Møller AP, Birkhead TR (1994) The evolution of plumage brightness in birds is related to extrapair paternity. Evolution 48:1089–1100
Morales J, Velando A, Moreno J (2008) Pigment allocation to eggs decreases plasma antioxidants in a songbird. Behav Ecol Sociobiol 63:227–233
Moreno J (1989) Body-mass variation in breeding northern wheatears: a field experiment with supplementary food. Condor 91:178–186
Moreno J, Gil D, Cantarero A, López-Arrabé J (2014) Extent of a white plumage patch covaries with testosterone levels in female pied flycatchers Ficedula hypoleuca. J Ornithol 155:639–648
Moreno J, Lobato E, González-Braojos S, Ruiz-de-Castañeda R (2010a) Nest construction costs affect nestling growth: a field experiment in a cavity-nesting passerine. Acta Ornithol 45:139–145
Moreno J, Martínez J, Corral C, Lobato E, Merino S, Morales J, Martínez-de la Puente JM, Tomás G (2008) Nest construction rate and stress in female pied flycatchers Ficedula hypoleuca. Acta Ornithol 43:57–64
Moreno J, Martínez JG, González-Braojos S, Cantarero A, Ruiz-de-Castañeda R, Precioso M, López-Arrabé J (2015) Extra-pair paternity declines with female age and wing length in the pied flycatcher. Ethology. 121:501–512
Moreno J, Martínez JG, González-Braojos S, Ruiz-de-Castañeda R, Cantarero A, Sánchez-Tojar A (2013) Extra-pair matings, context-dependence and offspring quality: a brood manipulation experiment in pied flycatchers. Behaviour 150:359–380
Moreno J, Martínez JG, Morales J, Lobato E, Merino S, Tomás G, Vásquez RA, Möstl E, Osorno JL (2010b) Paternity loss in relation to male age, territorial behaviour and stress in the pied flycatcher. Ethology 116:76–84
Moreno J, Merino S, Lobato E, Ruiz-De-Castañeda R, Martínez-De La Puente J, Del Cerro S, Rivero-De Aguilar J (2009) Nest-dwelling ectoparasites of two sympatric hole-nesting passerines in relation to nest composition: an experimental study. Ecoscience 16:418–427
Moreno J, Merino S, Potti J, de León A, Rodríguez R (1999) Maternal energy expenditure does not change with flight costs or food availability in the pied flycatcher (Ficedula hypoleuca): costs and benefits for nestlings. Behav Ecol Sociobiol 46:244–251
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Norberg RA (1981) Temporary weight decrease in breeding birds may result in more fledged young. Am Nat 118:838–850
Pennycuick CJ (1982) The flight of petrels and albatrosses (Procellariiformes), observed in South Georgia and its vicinity. Philos Trans R Soc B 300:75–106
Petrie M, Kempenaers B (1998) Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol Evol 13:52–58
Pilastro A, Griggio M, Biddau L, Mingozzi T (2002) Extrapair paternity as a cost of polygyny in the rock sparrow: behavioural and genetic evidence of the ‘trade-off’ hypothesis. Anim Behav 63:967–974
Plaza M, Cantarero A, Cuervo JJ, Moreno J (2018) Female incubation attendance and nest vigilance reflect social signaling capacity: a field experiment. Behav Ecol Sociobiol 72:24
Plaza M, Cantarero A, Gil D, Moreno J (2019) Experimentally flight-impaired females show higher levels of extra-pair paternity in the pied flycatcher Ficedula hypoleuca. Biol Lett 15:20190360
Ramos AG, Nunziata SO, Lance SL, Rodríguez C, Faircloth BC, Gowaty PA, Drummond H (2014) Interactive effects of male and female age on extra-pair paternity in a socially monogamous seabird. Behav Ecol Sociobiol 68:1603–1609
Ruiz-de-Castañeda R, Burtt EH, González-Braojos S, Moreno J (2012) Bacterial degradability of an intrafeather unmelanized ornament: a role for feather-degrading bacteria in sexual selection? Biol J Linn Soc 105:409–419
Salewski V, Hochachka WM, Flinks H (2014) Changes in stonechat Saxicola torquata morphology: a response to climate change? J Ornithol 155:601–609
Sanz JJ, Moreno J (1995) Mass loss in brooding female pied flycatchers Ficedula hypoleuca: no evidence for reproductive stress. J Avian Biol 26:313–320
Sirkiä PM, Adamík P, Artemyev AV et al (2015) Fecundity selection does not vary along a large geographical cline of trait means in a passerine bird. Biol J Linn Soc 114:808–827
Slagsvold T, Dale S (1996) Disappearance of female pied flycatchers in relation to breeding stage and experimentally induced molt. Ecology 77:461–471
Stutchbury BJ, Robertson RJ (1987) Behavioral tactics of subadult female floaters in the tree swallow. Behav Ecol Sociobiol 20:413–419
Stutchbury BJ, Morton ES (1995) The effect of breeding synchrony on extra-pair mating systems in songbirds. Behaviour 132:675–690
Tomás G, Merino S, Moreno J, Sanz JJ, Morales J, García-Fraile S (2006) Nest weight and female health in the blue tit (Cyanistes caeruleus). Auk 123:1013–1021
van den Hout PJ, Mathot KJ, Maas LRM, Piersma T (2010) Predator escape tactics in birds: linking ecology and aerodynamics. Behav Ecol 21:16–25
Videler JJ (2005) Avian flight. Oxford University Press, Oxford
Von Haartman L (1956) Territory in the pied flycatcher. Ibis 98:460–475
Westneat DF, Stewart IRK (2003) Extra-pair paternity in birds: causes, correlates, and conflict. Annu Rev Ecol Evol Sci 34:365–396
Acknowledgements
This study is a contribution to the research developed at “Ventorrillo” field station. We are very grateful to D. Gil for improving the manuscript with his comments and to A. Machordom for helping at the molecular Lab of Museo Nacional de Ciencias Naturales. We are also grateful to the referees, whose feedback substantially improved the manuscript.
Funding
This study was financed by project CGL2013-48193-C3-3-P and CGL2017-83843-C2-1-P to JM from Spanish ‘Ministerio de Ciencia, Innovación y Universidades’. MP was supported by FPI grant from ‘Ministerio de Ciencia, Innovación y Universidades’. AC is supported by a postdoctoral fellowship from Fundación Ramón Areces.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MP has received research FPI grant from ‘Ministerio de Ciencia, Innovación y Universidades’. AC declares that he has no conflict of interest. JM declares that he has no conflict of interest.
Ethical approval
We were legally authorized to capture and handle pied flycatchers by Consejería de Medio Ambiente de Castilla y León (competent regional authority, protocol number EP/SG/706/2016, according to Royal Decree 53/2013), and by J. Donés, director of “Centro Montes de Valsaín”, to work in the study area. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experiments comply with current Spanish laws, and grant holder and field researchers were officially licensed for animal manipulation following current EU regulations on animal manipulation (authorization types C and D with reference numbers CAP-T-0123-15 and CAP-T-0121-15). The study was ethically approved by the Ethical Committee of the ‘Consejo Superior de Investigaciones Científicas’ (CSIC).
Additional information
Communicated by S. Pruett-Jones
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Plaza, M., Cantarero, A. & Moreno, J. An experimental increase in female mass during the fertile phase leads to higher levels of extra-pair paternity in pied flycatchers Ficedula hypoleuca. Behav Ecol Sociobiol 73, 161 (2019). https://doi.org/10.1007/s00265-019-2771-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2771-z