Abstract
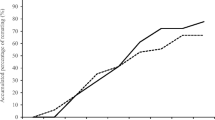
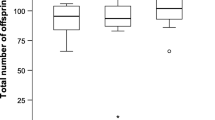
Polyandry is widespread among many animal taxa, yet the benefits for females are still debated. The two main hypotheses to explain its evolution are the direct benefits and the genetic benefits hypotheses, which are not mutually exclusive. We tested both in the wood tiger moth Arctia plantaginis (Arctiidae) by comparing fitness components in single and multiple mated females. We measured female longevity and number of eggs laid (i.e. direct benefits), as well as offspring hatching success and survival (i.e. genetic benefits). Fitness components did not differ between single and multiple mated females; therefore, there was no evidence to support either direct or genetic benefits hypotheses, or any costs. After paternity analyses, we obtained qualitatively similar results by comparing clutches effectively sired by one male with clutches sired by two males, regardless of the number of times a female mated. We further investigated the proximate mechanisms driving the outcome of paternity patterns. First male precedence, last male precedence, and mixed paternity were present in equal proportions, although there was a trend towards last male sperm precedence in later clutches. Interestingly, in polyandric females, the age of the second male positively affected the number of eggs laid and the number of surviving offspring, indicating an advantage for older males, possibly due to a higher parental investment. We suggest in light of recent theoretical work that the acceptance of more partners in female A. plantaginis may have evolved to ensure fertilization and avoid the risk of virgin death.
Significance statement
Why do females mate with multiple males? Here, we investigate the effects of polyandry on female fitness components in the wood tiger moth Arctia plantaginis. We do not find any support for the direct or genetic benefits hypotheses, or any costs of polyandry. We do not find any clear paternity patterns as last male, first male, and mixed paternities are equally present. We suggest that in this species polyandry may have evolved to ensure fertilization and avoid the risk of virgin death.



Similar content being viewed by others
References
Alcock J, Thornhill R (1983) The evolution of insect mating systems. Harvard University Press, Cambridge
Arnqvist G (1989) Multiple mating in a water strider: mutual benefits or intersexual conflict? Anim Behav 38:749–756
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Bateman A (1948) Intrasexual selection. Heredity 2:349–368
Birkhead TR (2000) Promiscuity: an evolutionary history of sperm competition. Harvard University Press
Birkhead TR, and Moller AP (1992) Sperm competition in birds. Evolutionary causes and consequences. Academic Press, San Diego (CA)
Birkhead TR, Parker GA (1997) Sperm competition and mating systems. Pp. 121–45 in. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach, 4th edn. Blackwell Publishing Ltd, Oxford
Bretman A, Newcombe D, Tregenza T (2009) Promiscuous females avoid inbreeding by controlling sperm storage. Mol Ecol 18:3340–3345
Britz H, Wingfield BD, Coutinho TA, Wingfield MJ (2002) Sequence characterized amplified polymorphic markers for the pitch canker pathogen, Fusarium circinatum. Mol Ecol Notes 2:577–580
Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L (1995) Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373:241–244
Chargè R, Wedell N, Lindstedt C, Hämäläinen L, Övermark E, Mappes J (2016) Variation in male fertility in a polymorphic moth, Parasemia plantaginis. Anim Behav 111:33–40
Dougherty LR, Simmons LW, Shuker DM (2016) Postcopulatory sexual selection when a female mates once. Anim Behav 116:13–16
Dunlap-Pianka H, Boggs CL, and Gilbert LE (1977) Ovarian dynamics in heliconiine butterflies: programmed senescence versus eternal youth. Science 197(80-. ):487–90
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press
Egan AL, Hook KA, Reeve HK, Iyengar VK (2016) Polyandrous females provide sons with more competitive sperm: support for the sexy-sperm hypothesis in the rattlebox moth (Utetheisa ornatrix). Evolution 70:72–81
Evans JP, Zane L, Francescato S, Pilastro A (2003) Directional postcopulatory sexual selection revealed by artificial insemination. Nature 421:360–363
Fedorka KM, Mousseau TA (2002) Material and genetic benefits of female multiple mating and polyandry. Anim Behav 64:361–367
Fisher RA (1915) The evolution of sexual preference. Eugen Rev.
Fricke C, Maklakov AA (2007) Male age does not affect female fitness in a polyandrous beetle , Callosobruchus maculatus. Anim Behav 74:541–548
Galarza JA, Nokelainen O, Ashrafi R, Hegna RH, Mappes J (2014) Temporal relationship between genetic and warning signal variation in the aposematic wood tiger moth (Parasemia plantaginis). Mol Ecol 23:4939–4957
Galarza JA, Viinikainen SM, Ashrafi R, Mappes J (2011) First microsatellite panel for the wood tiger moth (Parasemia plantaginis). Conserv Genet Resour 3:197–199
Gelman A, Hill J (2006) Data analysis using regression and multilevel hierarchical models. Cambridge University Press, Cambridge
Gordon SP, Kokko H, Rojas B, Nokelainen O, Mappes J (2015) Colour polymorphism torn apart by opposing positive frequency-dependent selection, yet maintained in space. J Anim Ecol 84:1555–1564
Gwynne DT (1984) Courtship feeding increases female reproductive success in bushcrickets. Nature 307:361–363
Gwynne DT (2008) Sexual conflict over nuptial gifts in insects. Annu Rev Entomol 53:83–101
Hegna RH, Nokelainen O, Hegna JR, Mappes J (2013) To quiver or to shiver: increased melanization benefits thermoregulation, but reduces warning signal efficacy in the wood tiger moth. Proc Biol Sci 280:20122812
Holman L, Kokko H (2013) Extinction risk and conservation the consequences of polyandry for population viability, the consequences of polyandry for population viability, extinction risk and conservation. Phil Trans R Soc B 368:20120053
Ivy TM, Sakaluk SK (2005) Polyandry promotes enhanced offspring survival in decorated crickets. Evolution 59:152–159
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev Camb Philos Soc 75:21–64
Jones TM, Elgar MA (2004) The role of male age, sperm age and mating history on fecundity and fertilization success in the hide beetle. Proc R Soc B Biol Sci 271(1545):1311–1318
Kehl T, Karl I, Fischer K (2013) Old-male paternity advantage is a function of accumulating sperm and last-male precedence in a butterfly. Mol Ecol 22:4289–4297
Knell RJ, Webberley KM (2004) Sexually transmitted diseases of insects: distribution, evolution, ecology and host behaviour. Biol Rev Camb Philos Soc 79:557–581
Kokko H, Mappes J (2013) Multiple mating by females is a natural outcome of a null model of mate encounters. Entomol Exp Appl 146:26–37
Kokko H, Mappes J (2005) Sexual selection when fertilization is not guaranteed. Evolution 59:1876–1885
LaMunyon CW (2001) Determinants of sperm precedence in a noctuid moth Heliothis virescens: a role for male age. Ecol Entomol 26:388–394
LaMunyon CW, Eisner T (1993) Postcopulatory sexual selection in an arctiid moth (Utetheisa ornatrix). Proc Natl Acad Sci U S A 90:4689–4692
Lindstedt C, Eager H, Ihalainen E, Kahilainen A, Stevens M, Mappes J (2011) Direction and strength of selection by predators for the color of the aposematic wood tiger moth. Behav Ecol 22:580–587
Lindstedt C, Lindström L, Mappes J (2008) Hairiness and warning colours as components of antipredator defence: additive or interactive benefits? Anim Behav 75:1703–1713
Lindstedt C, Morehouse N, Pakkanen H, Casas J, Christides J-P, Kemppainen K, Lindström L, Mappes J (2010) Characterizing the pigment composition of a variable warning signal of Parasemia plantaginis larvae. Funct Ecol 24:759–766
Magurran AE, Seghers BH (1994) A cost of sexual harassment in the guppy, Poecilia reticulata. Proc R Soc B 258:89–92
Milonas PG, Andow DA (2010) Virgin male age and mating success in Ostrinia nubilalis ( Lepidoptera : Crambidae ). Anim Behav 79:509–514
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Nokelainen O, Hegna RH, Reudler JH, Lindstedt C, Mappes J (2012) Trade-off between warning signal efficacy and mating success in the wood tiger moth. Proc Biol Sci 279:257–265
Ojala K, Lindström L, Mappes J (2007) Life-history constraints and warning signal expression in an arctiid moth. Funct Ecol 21:1162–1167
Osikowski A, Rafin’ski J (2001) Multiple insemination increases reproductive success of female Montandon’s newt ( Triturus montandoni , Caudata, Salamandridae). Behav Ecol Sociobiol 49:145–149
Parker GA (1970) Sperm competition and its evolutionary consequences in the insects. Biol Rev 45:525–567
Parker GA (1984) Sperm competition and the evolution of animal mating strategies. P. 1/60 in Sperm competition and the evolution of animal mating systems
Parker GA (1982) Why are there so many tiny sperm ? Sperm competition and the maintenance of two sexes. J Theor Biol 96:281–294
Parker GA, Birkhead TR (2013) Polyandry: the history of a revolution. Philos Trans R Soc B Biol Sci 368:20120335–20120335
Pizzari T, Wedell N (2013) The polyandry revolution. Philos Trans R Soc B Biol Sci 368:20120041–20120041
R Development Core Team (2013) R: A language and environment for statistical Computing R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Ridley M (1988) Mating frequency and fecundity in insects. Biol Rev 63:509–549
Rojas B, Gordon SP, Mappes J (2015) Frequency-dependent flight activity in the colour polymorphic wood tiger moth. Curr Zool 61:762–772
Shuker DM, and Simmons LW (2014) The evolution of insect mating systems. Oxford University Press, Oxford
Simmons LW (2001) Sperm competition and its evolutionary consequences in the insects. Princeton University Press
Simmons LW (2005) The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu Rev Ecol Evol Syst 36:125–146
Simmons LW, Siva-Jothy MT (1998) Sperm competition in insects: mechanisms and the potential for selection. In: Sperm competition and sexual selection, pp 341–434
Slatyer RA, Mautz BS, Backwell PRY, Jennions MD (2012) Estimating genetic benefits of polyandry from experimental studies: a meta-analysis. Biol Rev 87:1–33
Snook RR (2014) The evolution of polyandry. Pp. 159–180 in The evolution of insect mating systems
South A, Lewis SM (2011) The influence of male ejaculate quantity on female fitness: a meta-analysis. Biol Rev 86:299–309
Stearns SC (1992) The evolution of life histories. Oxford Univ. Press, New York
Svärd L, Wiklund C (1988) Fecundity, egg weight and longevity in relation to multiple matings in females of the monarch butterfly. Behav Ecol Sociobiol 23:39–43
Svensson M (1996) Sexual selection in moths: the role of chemical communication. Biol Rev 71:113–135
Taggart JB (2007) FAP: an exclusion-based parental assignment program with enhanced predictive functions: program note. Mol Ecol Notes 7:412–415
Taylor ML, Price TAR, Wedell N (2014) Polyandry in nature: a global analysis. Trends Ecol Evol 29:376–383 Elsevier Ltd
Teder T, Vellau H, Tammaru T (2014) Age and size at maturity: a quantitative review of diet-induced reaction norms in insects. Evolution 68:3217–3228
Tregenza T, Wedell N (2000) Genetic compatibility, mate choice and patterns of parentage: invited review. Mol Ecol 9:1013–1027
Tregenza T, Wedell N (2002) Polyandrous females avoid costs of inbreeding. Nature 415:71–73
Trivers RL (1972) Parental investment and sexual selection. P. in Sexual selection and the descent of man. Biological Laboratories, Harvard University, Cambridge, MA
Tuni C, Albo MJ, Bilde T (2013) Polyandrous females acquire indirect benefits in a nuptial feeding species. J Evol Biol 26:1307–1316
Vahed K (1998) The function of nuptial feeding in insects: a review of empirical studies. Biol Rev Camb Philos Soc 73:43–78
Wedell N (2005) Female receptivity in butterflies and moths. J Exp Biol 208:3433–3440
Wedell N, Cook PA (1998) Determinants of paternity in a butterfly. Proc R Soc B Biol Sci 265:625–630
Wiklund C, Forsberg J (1986) Courtship and male discrimination between virgin and mated females in the orange tip butterfly Anthocharis cardamines. Anim Behav 34:328–332
Wiklund C, Kaitala A, Wedell N (1998) Decoupling of reproductive rates and parental expenditure in a polyandrous butterfly. Behav Ecol 9:20–25
Wiklund C, Karlsson B, Leimar O (2001) Sexual conflict and cooperation in butterfly reproduction: a comparative study of polyandry and female fitness. Proc R Soc B Biol Sci 268:1661–1667
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of lack ‘s principle. Am Nat 100:687–690
Xu J, Wang Q (2009) A polyandrous female moth discriminates against previous mates to gain genetic diversity. Anim Behav 78:1309–1315
Yasui Y (1998) The “genetic benefits” of female multiple mating reconsidered. Trends Ecol Evol 13:246–260
Zeh JA, Zeh DW (1996) The evolution of polyandry I: intragenomic conflict and genetic incompatibility. Proc R Soc B Biol Sci 263:1711–1717
Acknowledgments
We would like to thank Kaisa Suisto for the maintenance of laboratory stock, Sari Viinikainen for the invaluable help with the molecular work, and Carol Gilsenan for the help with the experiments. We also thank Franzi Korner-Nievergelt for statistical advice, and Ossi Nokelainen, Remi Chargè, Federica Poli, and Cristina Tuni for the feedback.
Funding
This project was funded by the Centre of Excellence in Biological Interaction, via the Academy of Finland (Project No. 252411). FS was supported by an Erasmus scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. J. Hosken
Electronic supplementary material
Table S1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Santostefano, F., Galarza, J.A. & Mappes, J. Testing the direct and genetic benefit hypotheses of polyandry in the wood tiger moth. Behav Ecol Sociobiol 72, 109 (2018). https://doi.org/10.1007/s00265-018-2525-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2525-3