Abstract
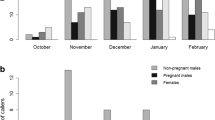
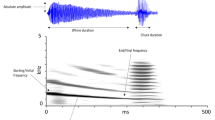
In many species that use acoustic signals for mate attraction, males are usually the most vocal sex. In frogs, females typically remain silent, while males produce advertisement calls to attract mates. In some species, females vocalize, but usually as a response to an initial male advertisement call. The smooth guardian frog (Limnonectes palavanensis), found on Borneo, has exclusive paternal care while the females mate and desert after laying the clutch. Males provide care to the eggs until hatching and then they transport the tadpoles to small bodies of water. The vocal repertoire of this species has never been described. Males have a distinctive advertisement call to attract females, but produce the call very infrequently. We found that females of L. palavanensis not only respond to male advertisement calls but also vocalize spontaneously, forming lek-like aggregations around a single male. Males may or may not respond to a particular female with a short courtship call, which is elicited only by the female call and not the male advertisement call. The calling rate of females is consistently higher throughout the night compared with the calling rate of males. These observations suggest that this species exhibits a reversal in calling behavior and possibly a sex-role-reversed mating system.
Significance statement
Exceptional cases of species with a sex-role reversed mating system have been observed in fishes and birds, but not in frogs. For sex-role reversal to occur, there must be intense parental care by the males and a surplus of females. Additionally, females should exhibit characteristics that are usually observed in males in species with conventional sex roles. We found that in L. palavanensis, females are highly vocal, exhibiting higher calling rates compared with the calling rates of the males. This behavior, where females out-signal males has not been observed in anurans. This female calling behavior coupled with observations of several females approaching a male provides evidence of a female-biased operational sex ratio, a characteristic of a sex-role-reversed mating system. Thus, this study provides quantitative evidence that L. palavanensis exhibits various aspects consistent with a sex-role reversed mating system.







Similar content being viewed by others
References
Andersson M (1995) Evolution of reversed sex roles, sexual size dimorphism, and mating system in coucals (Centropodidae, Aves). Biol J Linn Soc 54:173–181
Bee MA, Suyesh R, Biju SD (2013) Vocal behavior of the Ponmudi bush frog (Raorchestes graminirupes): repertoire and individual variation. Herpetologica 69:22–35
Berglund A (1995) Many mates make male pipefish choosy. Behaviour 132:213–218
Berglund A, Rosenqvist G (1993) Selective males and ardent females in pipefishes. Behav Ecol Sociobiol 32:331–336
Boistel R, Sueur J (1997) Comportement sonore de la femelle de Platymantis vitiensis (Amphibia, Anura) en l’absence du mâle. CR Acad Sci Sci III-Vie 320:933–941
Boistel R, Sueur J (2002) XVIIth symposium of the international bioacoustic council—abstracts. Bioacoustics 13:77–102
Bosch J (2001) Female reciprocal calling in the Iberian midwife toad (Alytes cisternasii) varies with male call rate and dominant frequency: implications for sexual selection. Naturwissenschaften 88:434–438
Bush SL (1996) Why is double clutching rare in the Majorcan midwife toad? Anim Behav 52:913–922
Bush SL (1997) Vocal behavior of males and females in the Majorcan midwife toad. J Herpetol 31:251–257
Bush SL, Bell DJ (1997) Courtship and female competition in the Majorcan midwife toad, Alytes muletensis. Ethology 103:292–303
Bush SL, Dyson ML, Halliday TR (1996) Selective phonotaxis by males in the Majorcan midwife toad. Proc R Soc Lond B 263:913–917
Cooley JR, Marshall DC (2001) Sexual signaling in periodical cicadas, Magicicada spp. (Hemiptera: Cicadidae). Behaviour 138:827–855
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Ekstrom JMM, Burke T, Randrianaina L, Birkhead TR (2007) Unusual sex roles in a highly promiscuous parrot: the greater vasa parrot Caracopsis vasa. Ibis 149:313–320
Emerson SB (1992) Courtship and nest-building behavior of a Bornean frog, Rana blythii. Copeia 1992:1123–1127
Emerson SB, Inger RF, Iskandar DT (2000) Molecular systematics and biogeography of the fanged frogs of Southeast Asia. Mol Phylogenet Evol 16:131–142
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Emlen ST, Wrege PH, Webster MS (1998) Cuckoldry as a cost of polyandry in the sex-role reversed jacana, Jacana jacana. Proc R Soc Lond B 265:2359–2364
Geberzahn N, Goymann W, Muck C, ten Cate C (2009) Females alter their song when challenged in a sex-role reversed bird species. Behav Ecol Sociobiol 64:193–204
Geberzahn N, Goymann W, ten Cate C (2010) Threat signaling in female song—evidence from playbacks in a sex-role reversed bird species. Behav Ecol 21:1147–1155
Gerhardt HC (1991) Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim Behav 42:615–635
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. The University of Chicago Press, Chicago
Given MF (1993) Male response to female vocalization in the carpenter frog, Rana virgatipes. Herpetologica 44:304–317
Goyes Vallejos J (2016) Parental care and acoustic communication of the smooth guardian frog Limnonectes palavanensis, a Bornean frog with possible sex-role reversal. PhD Dissertation, University of Connecticut
Goymann W, Wittenzellner A, Wingfield J (2004) Competing females and caring males. Sex-role reversal in African black coucals, Centropus grillii. Anim Behav 68:733–740
Grafe TU, Bitz JH (2004) Functions of duetting in the tropical boubou, Laniarius aethiopicus: territorial defence and mutual mate guarding. Anim Behav 68:193–201
Hauselberger KF, Alford RA (2005) Effects of season and weather on calling in the Australian microhylid frog Austrochaperina robusta. Herpetologica 61:349–363
Illes AE, Yunes-Jimenez L (2009) A female songbird out-sings male conspecifics during simulated territorial intrusions. Proc R Soc Lond B 276:981–986
Inger RF, Voris HK (1988) Taxonomic status and reproductive biology of Bornean tadpole-carrying frogs. Copeia 1988:1060–1061
Inger RF, Voris HK, Walker P (1986) Larval transport in a Bornean ranid frog. Copeia 1986:523–525
Judge KA, Swanson SJ, Brooks RJ (2000) Rana catesbeiana (Bullfrog): female vocalization. Herpetol Rev 31:236–237
Kaefer IL, Montanarin A, da Costa RS, Lima AP (2012) Temporal patterns of reproductive activity and site attachment of the brilliant-thighed frog Allobates femoralis from Central Amazonia. J Herpetol 46:549–554
Kokko H, Jennions MD (2008) Parental investment, sexual selection, and sex ratios. J Evol Biol 21:919–948
Krishna S, Krishna S (2005) Female courtship calls of the litter frog (Rana curtipes) in the tropical forests of Western Ghats, South India. Amphibia-Reptilia 26:431–435
Marquez R, Verrell PA (1991) The courtship and mating of the Iberian midwife toad Alytes cisternasii (Amphibia: Anura: Discoglossidae). J Zool 225:125–139
Matsui M (1995) Calls produced by a “voiceless” frog, Rana blythi Boulenger 1920, from Peninsular Malaysia (Amphibia, Anura). Trop Zool 8:325–331
Monteiro N, Carneiro D, Antunes A, Queiroz N, Vieira M, Jones A (2017) The lek mating system of the worm pipefish (Nerophis lumbriciformis): a molecular maternity analysis and test of the phenotype-linked fertility hypothesis. Mol Ecol 26:1371–1385
Orlov NL (1997) Breeding behavior and nest construction in a Vietnam frog related to Rana blythi. Copeia 1997:464–465
Pettitt BA, Bourne GR, Bee MA (2013) Advertisement call variation in the golden rocket frog (Anomaloglossus beebei): evidence for individual distinctiveness. Ethology 119:244–256
Price JJ, Yunes-Jimenez L, Osorio-Beristain M (2008) Sex-role reversal in song? Females sing more frequently than males in the streak-backed oriole. Condor 110:387–392
Price JJ, Lanyon SM, Omland KE (2009) Losses of female song with changes from tropical to temperate breeding in the New World blackbirds. Proc R Soc Lond B 276:1971–1980
Rebar D, Bailey NW, Zuk M (2009) Courtship song’s role during female mate choice in the field cricket Teleogryllus oceanicus. Behav Ecol 20:1307–1314
Rodríguez RL, Sullivan LE, Cocroft RB (2004) Vibrational communication and reproductive isolation in the Enchenopa binotata species complex of treehoppers (Hemiptera: Membracidae). Evolution 58:571–578
Roithmair ME (1994) Male territoriality and female mate selection in the dart-poison frog Epipedobates trivittatus (Dendrobatidae, Anura). Copeia 1994:107–115
Rowley JJL, Le DTT, Hoang HD, Altig R (2014) The breeding behaviour, advertisement call and tadpole of Limnonectes dabanus (Anura: Dicroglossidae). Zootaxa 3881:195–200
Searcy WA, Nowicki S (2005) The evolution of animal communication: reliability and deception in signaling systems. Princeton University Press, Princeton, NJ
Shen JX, Feng AS, Xu ZM, Yu ZL, Arch VS, Yu XJ, Narins PM (2008) Ultrasonic frogs show hyperacute phonotaxis to female courtship calls. Nature 453:914–916
Slater PJB, Mann NI (2004) Why do the females of many bird species sing in the tropics? J Avian Biol 35:289–294
Smith RL (1979) Paternity assurance and altered roles in the mating behaviour of a giant water bug, Abedus herberti (Heteroptera: Belostomatidae). Anim Behav 27:716–725
Summers K (1989) Sexual selection and intra-female competition in the green poison-dart frog, Dendrobates auratus. Anim Behav 37:797–805
Suthers RA, Narins PM, Lin WY, Schnitzler HU, Denzinger A, Xu CH, Feng AS (2006) Voices of the dead: complex nonlinear vocal signals from the larynx of an ultrasonic frog. J Exp Biol 209:4984–4993
Tobias ML, Viswanathan SS, Kelley DB (1998) Rapping in the African clawed frog Xenopus laevis. P Natl Acad Sci USA 95:1870–1875
Toledo LF, Martins IA, Bruschi DP, Passos MA, Alexandre C, Haddad CFB (2014) The anuran calling repertoire in the light of social context. Acta Ethol 18:87–99
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine-Atherton, Chicago, pp 136–179
Ursprung E, Ringler M, Jehle R, Hödl W (2011) Strong male/male competition allows for nonchoosy females: high levels of polygynandry in a territorial frog with paternal care. Mol Ecol 20:1759–1771
Vincent ACJ (1992) Prospects for sex role reversal in teleost fishes. Neth J Zool 42:392–399
Wagner WE Jr, Reiser MG (2000) The importance of calling song and courtship song in female mate choice in the variable cricket. Anim Behav 59:1219–1226
Wells KD (1977) The social behaviour of anuran amphibians. Anim Behav 25:666–693
Wells KD (1978) Courtship and parental behavior in a Panamanian poison-arrow frog, Dendrobates auratus. Herpetologica 34:148–155
Wells KD (1980) Social behavior and communication of a dendrobatid frog (Colostethus trinitatis). Herpetologica 36:189–199
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Wells MM, Henry CS (1992) The role of courtship songs in reproductive isolation among populations of green lacewings of the genus Chrysoperla (Neuroptera: Chrysopidae). Evolution 46:31–42
Wells KD, Taigen TL (1986) The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav Ecol Sociobiol 19:9–18
Zimmerman BL, Bogart JP (1984) Vocalizations of primary forest frog species in the Central Amazon. Acta Amaz 14:473–519
Acknowledgments
We are thankful to the director of the Institute for Biodiversity and Environmental Research (IBER), the director of the Kuala Belalong Field Studies Centre (KBFSC) Hajah Masnah binti Haji Mirasan, and Rodzay bin Haji Abdul Wahab for facilitating our field work. We thank Teddy Chua Wee Li and Muhammad Salleh bin Abdullah Bat as well as the staff at the KBFSC for the logistic support in the field. We are grateful to Jana Englmeir, Fiana Shapiro, and Alexander Terry for field work assistance. We thank Morgan Tingley and Robert Bagchi for invaluable statistical advice and Elizabeth Jockusch, Mark Urban, Charles Henry, and two anonymous reviewers for helpful comments that greatly improved the manuscript. This work was partially funded by the Ralph M. Wetzel Endowment Fund and the Ecology and Evolutionary Biology Department at the University of Connecticut. Original recordings and datasets associated with this paper are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
JGV designed the study, collected and analyzed the data, and drafted the manuscript. TUG helped with the experimental design and data collection and provided comments on the manuscript. HHAS facilitated fieldwork. KDW helped with the experimental design of the study, drafting of the manuscript, and provided funding for fieldwork.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All the behavioral observations and experimental methods done in this study were done in accordance with the Institutional Animal Care and Use Committee at the University of Connecticut (Approved Protocol No. A12-028) and followed the guidelines of the Animal Behavior Society (ABS) for the treatment of animals in behavioral research.
Additional information
Communicated by K. Summers
Electronic supplementary material
ESM 1
(MP4 40905 kb)
Rights and permissions
About this article
Cite this article
Goyes Vallejos, J., Ulmar Grafe, T., Ahmad Sah, H.H. et al. Calling behavior of males and females of a Bornean frog with male parental care and possible sex-role reversal. Behav Ecol Sociobiol 71, 95 (2017). https://doi.org/10.1007/s00265-017-2323-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2323-3