Abstract
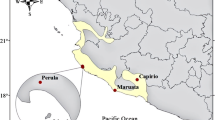
Interpopulational variation in sexual signals may lead to premating reproductive isolation and eventually may result in speciation. We explored the role of chemical cues secreted by the femoral glands of male lizards Psammdoromus algirus in chemosensory recognition between two distinct genetic lineages from Central Spain. We hypothesized that if there were differences in chemical sexual signals between lineages, these may result in differential recognition and mate preferences. This might lead to reproductive isolation, which would allow the observed morphological and genetic differences between lineages. Our results showed that males of each lineage secreted a singular mixture of compounds in their femoral secretions. However, females were apparently not able to discriminate the lineage of males by chemosensory cues or, alternatively, this discrimination may not be important for females. Moreover, females did not select or reject areas scent marked by males of their own vs. the other lineage. However, previous studies suggest that females might prefer scent of males with particular chemical characteristics that show interindividual variability but do not vary between lineages. Similarly, males did not discriminate between the scents of females of the two lineages, although they had greater chemosensory responses to scents of larger females. In contrast, males clearly discriminated the lineage of other males based on their scents alone, showing chemosensory and aggressive responses that were higher to scents of males of the other lineage. If males of the opposite lineage were more prone to be detected and excluded from a male territory due to their differences in chemical signals, this may probably impede the access of males of one lineage to females of the opposite lineage. This might result in reproductive isolation between lineages. We suggest that the current genetic divergence observed between lineages of P. algirus lizards may be mediated by intrasexual relationships among males, but not by female mate preferences. Significance statement Sexual signals often vary geographically to maximize their efficiency in communication under local conditions. Such variation may, however, affect recognition between individuals of different populations, resulting in reproductive isolation and speciation. We studied two populations (lineages) of a lizard with genetic and morphological differences. We found clear inter-lineage variation in chemical profiles of sexual signals of males. However, females did not recognize these differences by chemosensory cues and did not prefer or reject areas scent-marked by males of the two lineages. In contrast, males recognized and responded more aggressively toward scent of males of the opposite lineage. This might impede access of males of one lineage to females of the other. We suggest that the observed differences between lineages may result from partial reproductive isolation, which can be mediated by agonistic interactions between males rather than by female mate preferences.




Similar content being viewed by others
References
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325
Alberts AC (1992) Constraints on the design of chemical communication systems in terrestrial vertebrates. Am Nat 139:62–89
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E Ltd, Plymouth, UK
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Aragón P, López P, Martín J (2001) Chemosensory discrimination of familiar and unfamiliar conspecifics by lizards: implications of field spatial relationships between mles. Behav Ecol Sociobiol 50:128–133
Barbosa D, Font E, Desfilis E, Carretero MA (2006) Chemically mediated species recognition in closely related Podarcis wall lizards. J Chem Ecol 32:1587–1598
Boughman JW (2001) Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411:944–948
Boughman JW (2002) How sensory drive can promote speciation. Trends Ecol Evol 17:571–577
Carazo P, Font E, Desfilis E (2007) Chemosensory assessment of rival competitive ability and scent mark function in a lizard (Podarcis hispanica). Anim Behav 74:895–902
Carazo P, Font E, Desfilis E (2008) Beyond ‘nasty neighbours’ and ‘dear enemies’? Individual recognition by scent marks in a lizard (Podarcis hispanica). Anim Behav 76:1953–1963
Carranza S, Harris DJ, Arnold EN, Batista V, Gonzalez De La Vega JP (2006) Phylogeography of the lacertid lizard, Psammodromus algirus, in Iberia and across the Strait of Gibraltar. J Biogeogr 33:1279–1288
Carretero MA (2002) Sources of colour pattern variation in mediterranean Psammodromus algirus. Neth J Zool 52:43–60
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E Ltd, Plymouth, UK
Cooper WE (1994) Chemical discrimination by tongue-flicking in lizards: a review with hypotheses on its origin and its ecological and phylogenetic relationships. J Chem Ecol 20:439–487
Cooper WE (1998) Evaluation of swab and related tests as a bioassay for assessing responses by squamate reptiles to chemical stimuli. J Chem Ecol 24:841–866
Cooper WE, Burghardt GM (1990) A comparative analysis of scoring methods for chemical discrimination of prey by squamate reptiles. J Chem Ecol 16:45–65
Cooper WE, Vitt LJ (1986) Interspecific odour discrimination among syntopic congeners in Scincid lizards (genus Eumeces). Behaviour 97:1–9
Díaz JA (1993) Breeding coloration, mating opportunities, activity, and survival in the lacertid lizard Psammodromus algirus. Can J Zool 71:1104–1110
Díaz JA, Alonso-Gómez AL, Delgado MJ (1994) Seasonal variation of gonadal development, sexual steroids, and lipid reserves in a population of the lizard Psammodromus algirus. J Herpetol 28:199–205
Díaz JA, Iraeta P, Verdú-Ricoy J, Siliceo I, Salvador A (2012) Intraspecific variation of reproductive traits in a Mediterranean lizard: clutch, population, and lineage effects. Evol Biol 39:106–115
Díaz JA, Verdú-Ricoy J, Iraeta P, Llanos-Garrido A, Pérez-Rodríguez A, Salvador A (2016) There is more to the picture than meets the eye: adaptation for crypsis blurs phylogeographic structure in a lizard. J Biogeogr (in press)
Fox SF, Shipman PA (2003) Social behavior at high and low elevations: environmental release and phylogenetic effects in Liolaemus. In: Fox SF, McCoy JK, Baird TA (eds) Lizard social behavior. John Hopkins University Press, Baltimore, MD, pp. 310–355
Gabirot M, Castilla AM, López P, Martín J (2010a) Differences in chemical signals may explain species recognition between an island lizard, Podarcis atrata, and related mainland lizards, P. hispanica. Biochem Syst Ecol 38:521–528
Gabirot M, Castilla AM, López P, Martín J (2010b) Chemosensory species recognition may reduce the frequency of hybridization between native and introduced lizards. Can J Zool 88:73–80
Gabirot M, López P, Martín J (2012) Differences in chemical sexual signals may promote reproductive isolation and cryptic speciation between Iberian wall lizard populations. Int J Evol Biol 2012:698520
Gabirot M, López P, Martín J (2013) Female mate choice based on pheromone content may inhibit reproductive isolation between distinct populations of Iberian wall lizards. Curr Zool 59:210–220
Heathcote RJP, While GM, MacGregor HEA, Sciberras J, Leroy C, D’Ettorre P, Uller T (2016) Male behaviour drives assortative reproduction during the initial stage of secondary contact. J Evol Biol 29:1003–1015
Kopena R, López P, Martín J (2014) Relative contribution of dietary carotenoids and vitamin E to visual and chemical sexual signals of male Iberian green lizards: an experimental test. Behav Ecol Sociobiol 68:571–581
LeMaster MP, Mason RT (2003) Pheromonally mediated sexual isolation among denning populations of red-sided garter snakes, Thamnophis sirtalis parietalis. J Chem Ecol 29:1027–1043
López P, Amo L, Martín J (2006) Reliable signaling by chemical cues of male traits and health state in male lizards, Lacerta monticola. J Chem Ecol 32:473–488
López P, Gabirot M, Martín J (2009) Immune activation affects chemical sexual ornaments of male Iberian wall lizards. Naturwissenschaften 96:65–69
López P, Martín J (2002) Chemical rival recognition decreases aggression levels in male Iberian wall lizards, Podarcis hispanica. Behav Ecol Sociobiol 51:461–465
López P, Martín J (2005) Female Iberian wall lizards prefer male scents that signal a better cell-mediated immune response. Biol Lett 1:404–406
López P, Martín J (2011) Male iberian rock lizards may reduce the costs of fighting by scent-matching of the resource holders. Behav Ecol Sociobiol 65:1891–1898
López P, Martín J, Cuadrado M (2002) Pheromone mediated intrasexual aggression in male lizards, Podarcis hispanicus. Aggress Behav 28:154–163
López P, Martín J, Cuadrado M (2003) Chemosensory cues allow male lizards Psammodromus algirus to override visual concealment of sexual identity by satellite males. Behav Ecol Sociobiol 54:218–224
Martín J, Forsman A (1999) Social costs and development of nuptial coloration in male Psammodromus algirus lizards: an experiment. Behav Ecol 10:396–400
Martín J, López P (2000) Chemoreception, symmetry and mate choice in lizards. Proc R Soc Lond B 267:1265–1269
Martín J, López P (2006a) Interpopulational differences in chemical composition and chemosensory recognition of femoral gland secretions of male lizards Podarcis hispanica: implications for sexual isolation in a species complex. Chemoecology 16:31–38
Martín J, López P (2006b) Pre-mating mechanisms favoring or precluding speciation in a species complex: chemical recognition and sexual selection between types in the lizard Podarcis hispanica. Evol Ecol Res 8:643–658
Martín J, López P (2006c) Links between male quality, male chemical signals, and female mate choice in Iberian rock lizards. Funct Ecol 20:1087–1096
Martín J, López P (2006d) Vitamin D supplementation increases the attractiveness of males’ scent for female Iberian rock lizards. Proc R Soc Lond B 273:2619–2624
Martín J, López P (2006e) Age-related variation in lipophilic chemical compounds from femoral gland secretions of male lizards Psammodromus algirus. Biochem Syst Ecol 34:691–697
Martín J, López P (2007) Scent may signal fighting ability in male Iberian rock lizards. Biol Lett 3:125–127
Martín J, López P (2011) Pheromones and reproduction in reptiles. In: Norris DO, Lopez KH (eds) Hormones and reproduction of vertebrates, vol 3. Reptiles. Academic Press, San Diego, California, pp. 141–167
Martín J, López P (2012) Supplementation of male pheromone on rock substrates attracts female rock lizards to the territories of males: a field experiment. PLoS One 7:e30108
Martín J, López P (2013) Effects of global warming on sensory ecology of rock lizards: increased temperatures alter the efficacy of sexual chemical signals. Funct Ecol 27:1332–1340
Martín J, López P (2014) Pheromones and chemical communication in lizards. In: Rheubert JL, Siegel DS, Trauth SE (eds) The reproductive biology and phylogeny of lizards and tuatara. CRC Press, Boca Raton, Florida, pp. 43–77
Martín J, López P (2015) Condition-dependent chemosignals in reproductive behavior of lizards. Horm Behav 68:14–24
Martín J, Civantos E, Amo L, López P (2007a) Chemical ornaments of male lizards Psammodromus algirus may reveal their parasite load and health state to females. Behav Ecol Sociobiol 62:173–179
Martín J, Moreira PL, López P (2007b) Status-signalling chemical badges in male Iberian rock lizards. Funct Ecol 21:568–576
Martín J, Ortega J, López P (2015) Interpopulational variations in sexual chemical signals of Iberian wall lizards may allow maximizing signal efficiency under different climatic conditions. PLoS One 10:e0131492
Mas F, Jallon JM (2005) Sexual isolation and cuticular hydrocarbon differences between Drosophila santomea and Drosophila yakuba. J Chem Ecol 31:2747–2752
Mason RT (1992) Reptilian pheromones In: Gans C, Crews D (eds) Biology of the Reptilia, vol 18. University of Chicago Press, Chicago, pp. 114–228
Mason RT, Parker MR (2010) Social behavior and pheromonal communication in reptiles. J Comp Physiol A 196:729–749
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
Olsson M, Madsen T, Nordby J, Wapstra E, Ujvari B, Wittsell H (2003) Major histocompatibility complex and mate choice in sand lizards. Proc R Soc Lond B 270:S254–S256
Panhuis TM, Butlin R, Zuk M, Tregenza T (2001) Sexual selection and speciation. Trends Ecol Evol 16:364–371
Ritchie MG (2007) Sexual selection and speciation. Ann Rev Ecol Evol S 38:79–102
Runemark A, Gabirot M, Svensson EI (2011) Population divergence in chemical signals and the potential for premating isolation between islet- and mainland populations of the Skyros wall lizard (Podarcis gaigeae). J Evol Biol 24:795–809
Salvador A (2014) Psammodromus algirus (Linnaeus, 1758). In: Salvador A (ed) Reptiles. 2nd edn. Fauna Ibérica, Vol 10. Museo Nacional de Ciencias Naturales, CSIC, Madrid, 295–313
Salvador A, Veiga JP (2001) Male traits and pairing success in the lizard Psammodromus algirus. Herpetologica 57:77–86
Salvador A, Veiga JP, Martín J, López P (1997) Testosterone supplementation in subordinate small male lizards: consequences for aggressiveness, colour development, and parasite load. Behav Ecol 8:135–139
Salvador A, Veiga JP, Martín J, López P, Abelenda M, Puerta M (1996) The cost of producing a sexual signal: testosterone increases the susceptibility of male lizards to ectoparasitic infestation. Behav Ecol 7:145–150
Shine R, Reed RN, Shetty S, Lemaster M, Mason RT (2002) Reproductive isolating mechanisms between two sympatric sibling species of sea snakes. Evolution 56:1655–1662
Smadja C, Butlin RK (2009) On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102:77–97
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. WH Freeman, New York
Symonds MRE, Elgar MA (2008) The evolution of pheromone diversity. Trends Ecol Evol 23:220–228
Verdú-Ricoy J (2013) Origen y mantenimiento de la diversidad fenotípica en poblaciones ibéricas de lagartija colilarga. PhD Dissertation. Universidad Complutense, Madrid
Verdú-Ricoy J, Carranza S, Salvador A, Busack SD, Díaz JA (2010) Phylogeography of Psammodromus algirus (Lacertidae) revisited: systematic implications. Amphibia-Reptilia 31:576–582
Verdú-Ricoy J, Iraeta P, Salvador A, Díaz JA (2014) Phenotypic responses to incubation conditions in ecologically distinct populations of a lacertid lizard: a tale of two phylogeographic lineages. J Zool 292:184–191
Wyatt TD (2014) Pheromones and animal behaviour: chemical signals and signatures. Cambridge University Press, Cambridge
Acknowledgments
We thank T. Madsen and two anonymous reviewers for helpful comments and “El Ventorrillo” MNCN Field Station for use of their facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Captures and observations were performed under license from the Environmental Agency of Madrid Government (“Consejería del Medio Ambiente y Ordenación del Territorio de la Comunidad de Madrid”, Spain) and of Castilla la Mancha Government, and Patrimonio Nacional allowed access to El Pardo populations.
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent was not required.
Funding
Financial support was provided by the Spanish’s Ministerio de Economía y Competitividad projects MICIIN-CGL2010-17928/BOS and MINECO CGL2014-53523-P.
Additional information
Communicated by T. Madsen
Rights and permissions
About this article
Cite this article
Martín, J., López, P., Iraeta, P. et al. Differences in males’ chemical signals between genetic lineages of the lizard Psammodromus algirus promote male intrasexual recognition and aggression but not female mate preferences. Behav Ecol Sociobiol 70, 1657–1668 (2016). https://doi.org/10.1007/s00265-016-2171-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2171-6