Abstract
Purpose
The NexGen Legacy Posterior Stabilised (LPS) prosthesis (Zimmer Biomet, Warsaw, IN, USA) has augmentable and non-augmentable tibial baseplate options. We have noted an anecdotal increase in the number of cases requiring early revision for aseptic loosening since adopting the non-augmentable option. The purpose of this study was to ascertain our rates of aseptic tibial loosening for the two implant types within five years of implantation and to investigate the causes for any difference observed.
Methods
A database search was performed for all patients who underwent primary total knee arthroplasty (TKA) using the NexGen LPS between 2009 and 2015. Kaplan–Meier curves were plotted to assess for differences in revision rates between cohorts. We collected and compared data on gender, age, body mass index, component alignment and cement mantle quality as these were factors thought to affect the likelihood of aseptic loosening.
Results
Two thousand one hundred seventy-two TKAs were included with five year follow-up. There were 759 augmentable knees of which 14 were revised and 1413 non-augmentable knees of which 48 were revised. The overall revision rate at five years was 1.84% in the augmentable cohort and 3.4% in the non-augmentable cohort. The revision rate for aseptic loosening was 0.26% in the augmentable group and 1.42% in the non-augmentable group (p = 0.0241).
Conclusions
We have identified increased rates of aseptic loosening in non-augmentable components. This highlights the effect that minor implant changes can have on outcomes. We recommend that clinicians remain alert to implant changes and publish their own results when important trends are observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) remains the gold standard treatment for symptomatic arthritis of the knee and can significantly improve quality of life for the majority of patients; however, there are undoubtedly still problems related to this procedure which remain unresolved [1]. Since the advent of TKA in the 1970s, revision rates have declined due to a combination of refinement of surgical technique, implant design, fixation methods and improved antisepsis. Despite the decrease in revision rates, the absolute numbers of revision procedures continue to increase due to the increasing number of primary procedures [2, 3]. The most common reason cited for revision knee arthroplasty is aseptic loosening of the components, particularly on the tibial side [2, 4].
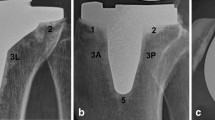
The NexGen Legacy Posterior Stabilised (LPS) prosthesis (Zimmer Biomet, Warsaw, IN, USA) was introduced to the market in 1997 and has been in use at our unit since 2007 for all primary TKA. This prosthesis has augmentable and non-augmentable options (Fig. 1); the augmentable option features 4 polyethylene lugs on the underside of the baseplate which can be removed to allow for attachment of augments and a polyethylene screw at the tip of the keel which can be replaced by a range of stems should these be required (Fig. 2). The simpler non-augmentable version has neither of these options and is used for the uncomplicated primary TKA (Fig. 3). Baseplates are available with 3° and 7° posterior slope stems. All of the tibial tray options are manufactured using an alloy of Titanium Ti-6-Al-4 V and incorporate a dove-tail locking mechanism for the polyethylene insert [5]. In addition, the NexGen tibial baseplate can be supplied pre-coated with polymethyl methacrylate (PMMA) and a non-pre-coated option is available [6].
Recent joint registry data from the UK reports an all cause revision rate for the NexGen of 2.17% at five years [2]. This includes both cruciate retaining and posterior stabilised designs and also a range of different tibial baseplates. Although registry data continues to become more sophisticated over time, there is insufficient detail within the data to ascertain specific rates of aseptic loosening for different implants within a family of implants at the current time.
Until early 2011, our institution used a 7° augmentable version of the tibial baseplate in all of our primary TKAs in order to keep a consistent inventory and reduce variation. In early 2011, this baseplate was gradually phased out in favour of a lower cost 7° non-augmentable option. At our institution, we have noted an anecdotal increase in the number of cases requiring early revision for aseptic loosening, particularly on the tibial side of the implant. The purpose of this study was to ascertain our observed rates of aseptic tibial loosening for the two implant types within five years of implantation and to investigate the causes for any difference observed. The hypothesis of this study was that there was an increased rate of aseptic tibial loosening in the non-augmentable component compared to the augmentable tibial component as a result of minor differences in the baseplate design.
Materials and methods
A retrospective search of a prospectively collated Bluespier® (Bluespier International Ltd, Worcestershire, UK) arthroplasty database was performed for all primary total knee arthroplasties (TKAs) performed between 05/01/2009 and 31/03/2015 in our institution with a minimum of five year follow-up. Our regional research and ethics committee were consulted prior to the study but formal ethical approval was not deemed necessary given that it included only a retrospective case note and radiograph review. The number of revision procedures was determined for this cohort during the five year period following implantation and the reason for revision was noted. The case notes for each patient who underwent revision were reviewed to ensure that the coded reason for revision was correct. The Musculoskeletal Infection Society (MSIS) criteria was used to determine whether infection was the cause for revision [7]. Patients were classed as having aseptic loosening if they had symptoms including pain, instability or swelling; had radiographic evidence of loosening; and did not meet the MSIS criteria for infection. Data was collected to indicate the date of revision surgery or date of mortality, age at time of surgery, date of original surgery and body mass index (BMI) for each patient.
We included all patients who underwent primary TKA using the NexGen LPS-fixed bearing prosthesis. All of the implants were posterior stabilised and were cemented without stems or augments. All of the augmentable tibial components had a PMMA pre-coating. The non-augmentable tibial components had no PMMA pre-coating. Palacos® R&G (Heraeus Medical, Hanau, Germany) cement was used until January 2012 until it was phased out over a two month period in order to reduce costs to Refobacin® (Zimmer Biomet, Warsaw, IN, USA). All procedures were performed by, or under the supervision of, one of the nine consultant orthopaedic surgeons within our institution. Over the study period, all surgeons that contributed to this study were high-volume specialists with an interest in knee arthroplasty. Technique for implantation was undertaken as per the operative technique recommended by the manufacturer although there were likely to be subtle differences in techniques utilised by each surgeon.
Post-operative radiographs for the included patients were reviewed to confirm each patient as having an augmentable baseplate (7° fluted stem) or a non-augmentable baseplate (7° fluted stem). An assessment of post-operative alignment and cement mantle quality was performed for all patients who had a non-augmentable baseplate and underwent revision for aseptic loosening. Radiographic views were performed in a standardised fashion by our radiology department but long-leg radiographs were not available. This radiographic assessment was carried out using a standardised technique described by Hampton et al. [8]. Coronal alignment of the femoral and tibial components was measured on the anteroposterior radiograph and sagittal tibial slope was measured on the lateral radiograph. The technique for cement mantle assessment is described in full within the original paper by Hampton et al. and involves counting the number of zones in which there is less than 2 mm of cement-bone penetration, counting radiolucent lines (RLL) and calculating the RLL as a percentage of the surface area available. To facilitate interpretation of these results, we performed the same analysis on a control group of non-augmentable knees which did not go on to require revision and a control group of augmentable knees which did not go on to require revision. The three control groups were selected from the database by searching for patients who were matched for age, BMI and gender as these factors could have an impact on revision rates. For completeness, we also assessed the cement mantle and alignment of the patients who had augmentable baseplates and underwent revision for aseptic loosening although this was a very small group (n = 2).
Statistical analysis was performed by an independent researcher using Graphpad Prism version 6. After sorting the primary TKAs into augmentable and non-augmentable cohorts, Kaplan–Meier curves were plotted to assess for differences in all revision rates and aseptic revision rates in both cohorts. Each cohort was also assessed to compare demographics which could potentially affect rates of aseptic loosening. Shapiro–Wilk test was used to determine normality of data and before parametric and non-parametric data was analysed using unpaired t tests and Mann–Whitney U tests, respectively. For comparisons containing more than two groups, parametric and non-parametric data was analysed using Fisher’s LSD, one-way ANOVA or Kruskal–Wallis ANOVA with Dunn’s post-test, respectively. Results were deemed significant with a p value of < 0.05.
Results
Overall, 2172 TKAs were performed in the study period with 759 augmentable tibial components inserted between 05/01/2009 and 01/2011 and 1413 non-augmentable tibial components between 01/2011 and 31/03/2015. During this 5-year follow-up, there were 14 revisions in the augmentable group and 48 revisions within the non-augmentable group (Table 1).
There was no difference in BMI or age between either tibial implant group (Table 2) although some data for BMI was missing (augmentable cohort, 445/759; non-augmentable cohort, 769/1413). BMI and age were both recorded at the time of primary surgery. Due to the low patient numbers, it is difficult to draw conclusive comparisons between augmentable and non-augmentable revisions; however, BMI alone does not appear to account for the differences in aseptic revisions observed. Furthermore, it appears non-augmentable aseptic loosening occurs in older patients compared to augmentable loosening although this does not reach statistical significance (p = 0.051).
The overall all cause revision rate at five years was 1.84% in the augmentable cohort and 3.4% in the non-augmentable cohort. At this same time point, the revision rate for aseptic loosening was 0.26% in the augmentable group and 1.42% in the non-augmentable group. The difference in overall revision rate for the two components was not statistically significant when analysed using the Kaplan–Meier analysis (Fig. 4). However, there was a significant difference (p = 0.0241) in implant failure due to aseptic tibial loosening requiring revision between the two cohorts (Fig. 5). The hazard ratio (and confidence intervals) between non-augmentable and augmentable implant is 2.9 (1.1 to 7.5) suggesting that aseptic loosening requiring revision is statistically more likely in the non-augmentable implant cohort than the augmentable cohort.
When assessing post-operative alignment of the non-augmentable group which went on to require revision, there was no significant difference in coronal alignment of the femoral or tibial components when compared to the two control groups. There was however a significant difference in the posterior slope between the aseptic loosening group (5.6°) and the non-augmentable control group (3.2°) (p = 0.02) and the augmentable control group (3.4°) (p = 0.03) (Table 3).
The cement mantle was assessed (Table 3) and an increased number of RLL were found in the non-augmentable aseptic loosening group (1.9) compared to the non-augmentable control group (0.8) (p = < 0.05) and the augmentable control group (0.3) (p = < 0.001). We also found a higher proportion of RLL as a percentage of the total surface area available in the non-augmentable aseptic loosening group (19.2%) compared to the non-augmentable control group (9.9%) (p = < 0.05) and the augmentable control group (6.6%) (p = < 0.01). There was no significant difference between any of the groups in terms of cement penetration and there was no significant difference between any of the measurements of alignment or cementation when comparing the two control groups. We have not performed statistical analysis on the alignment or cement mantle in the augmentable group aseptic loosening group because the patient numbers were so small (n = 2), anecdotally, though one of these knees showed an excellent cement mantle and one had several radiolucent lines.
Discussion
The purpose of this study was to ascertain differences in rates of aseptic loosening between augmentable and non-augmentable NexGen TKA tibial baseplates used in our unit. We confirmed our hypothesis of increased rates of aseptic loosening in non-augmentable components at five years (p = 0.0241) although it may not be possible to confirm that this was solely as a consequence of changes to implant design. There was no significant difference between the cohorts in terms of age or BMI which could account for this difference. We did observe some subtle differences in relation to the quality of the cement mantle in the non-augmentable knees which went on to aseptic loosening. The link between cement mantle quality and risk of aseptic loosening has already been shown [8]. In particular, we noted the consistent appearance of a RLL at the tip of the stem, at the implant cement interface of the non-augmentable components (Fig. 6). In the cases which went on to aseptic loosening, this line seems to progress and failure occurs at the implant cement interface. We observed less posterior slope in the knees which did not require revision comparing to those that went on to loosen aseptically. This was an unexpected finding. We exclusively use the 7° baseplates within our unit because increased posterior slope has been shown to increase post-operative range of flexion [9]. Our intra-operative cutting procedure is in keeping with the operative technique guidance from Zimmer Biomet using an extramedullary jig to match the native tibial alignment and a cutting block to build in the required 7° of posterior slope. It has previously been established that excessive slope leads to flexion instability and even early failure through detensioning of the collateral ligaments [10, 11]. This may offer some explanation for our findings but the overall numbers in the alignment comparison are small so we are reluctant to make any unjustified conclusions in this regard. The increased rates of RLL in the knees which went on to fail suggest that cementation technique may have played a role in the differences observed between our two groups. The effect of the PMMA pre-coating is also difficult to establish definitively. A previous study demonstrated lower rates of aseptic loosening in NexGen tibial baseplates which were non-pre-coated [12]. Conversely, a recent study raised concerns regarding rates of aseptic loosening in non-pre-coated baseplates although there was no direct comparison drawn with the pre-coated version [6]. In that context, it is difficult for us to ascertain the exact effect that the PMMA pre-coating had in our study but we can conclude that it may have contributed to the differences observed between our two groups.
There are several theories on the causes of aseptic loosening. As early as 1976, Harris noted loosening without infection in 4 hip arthroplasties [13]. His initial particle disease theory, which resulted in the development of uncemented implants [14, 15], did not have a positive effect on rates of aseptic loosening, which suggested that the mechanism is likely to be multifactorial. The effective joint space theory was first described in 1992 when it was hypothesised that polyethylene wear particles are dispersed into the effective joint space or that cement particles are generated by debonding or cement fracture [16]. According to this theory, access to the effective joint space is influenced by the cement/implant and cement/bone interfaces. Wear particles activate macrophages which release cytokines involved in bone remodelling such as prostaglandin E2, interleukin 1α, interleukin 6 and tumour necrosis factor α. These cytokines modulate osteoclast and osteoblast activity which results in osteolysis and an increase in the effective joint space [14, 17]. Macrophages may also differentiate into osteoclasts directly to further resorb bone tissue [18]. Stress shielding may also occur in the proximal tibia following total knee arthroplasty. The resultant osteopenia in the bone of the proximal tibia can allow micromotion of the implant which has been shown to result in failure [17]. There is also variation between individuals in the observed rates of osteolysis and it has been proposed that this may be secondary to an adverse cellular response [19]. Exactly, which factors predispose to aseptic loosening is still a matter of debate. Some authors have found an association with post-operative varus malalignment of the tibial component and increased BMI [20, 21]. Others have suggested that aseptic loosening occurs as a direct result of poor cement mantle quality [8]. With cementation being a key factor, some authors suggest that technical factors have the most influence over aseptic loosening rates [4]. But a systematic review was only able to relate an increased risk of aseptic loosening to male gender and high activity levels [22]. There have certainly been high rates of aseptic loosening observed with specific components which has led to a decrease in their usage [23].
Interpretation of our results in comparison to the literature is challenging. As previously noted, a number of different versions of the NexGen tibial baseplate are available, and in some studies, it can be difficult to determine exactly which version was being used [24, 25]. Similar problems are encountered when assessing arthroplasty registry data, and the cause of revision is often not clear [2]. Joint registry data for England and Wales has shown an all cause revision rate for the NexGen implant of 2.17% at five years [2]. This compares to our all cause revision rate of 2.85% at the same time point. The all cause revision rate for the non-augmentable tibial baseplate in our study however was 3.4% compared to a 1.84% rate in the augmentable group. This difference was not statistically significant (p = 0.0562). When we consider the rates of revision for aseptic loosening, it is useful to draw comparison to the results obtained by Arsoy et al. in 2013 [5]. In this study with similar methodology to our study, they observed a 2.2% revision rate for aseptic loosening at five years when assessing the 3° augmentable NexGen tibial baseplate. Our results compare favourably with these findings given that we found a revision rate for aseptic loosening of 0.26% at five years for the 7° augmentable baseplate and 1.42% for the non-augmentable 7° baseplate. Whilst our rates of revision for aseptic loosening in both groups compare favourably with previously reported rates, we have demonstrated an increased rate of revision for aseptic loosening at five years in the non-augmentable baseplate compared to the augmentable alternative. We have been unable to explain the difference observed when considering BMI or age but we accept that cementation technique may have played a role.
The strengths of this study include the overall patient numbers and the methodology. We also use the NexGen exclusively within our unit which ensures that all surgeons are familiar with it and there are no low-volume surgeons involved in this study in either time period.
There are a number of weaknesses in the study. When assessing alignment of the non-augmentable knees which failed due to aseptic loosening, we used short knee radiographs. Clearly, the use of long-leg alignment films would have been preferable but these were rarely available and the use of the short knee radiograph has been validated in previous studies [8]. BMI data was incomplete in our database but was present in similar proportions for both cohorts. We recognise that using revision as an endpoint for failure has its shortcomings and is likely to overestimate implant success. Furthermore, there is a small chance that there were some failures who underwent revision out with our local area or in the private sector. Evidence from the Scottish Arthroplasty Project in 2009 showed that the overall numbers of patients moving between health boards were small around the time of our study [26]. Furthermore, patients who undergo revision within Scotland are traced back to the hospital in which they underwent primary surgery when their data is collected for the Scottish Arthroplasty Project. Only patients who underwent revision surgery out with Scotland or within the private sector would have been missed in a national Picture Archiving and Communication System (PACS) search. Refobacin® cement (Zimmer Biomet, Warsaw, IN, USA) was only used in the non-augmentable cohort given the timing of the introduction of this product in our institution. It is possible therefore that this could have contributed to the aseptic loosening rate differences or indeed the radiographic differences within the cement mantle. In 2011 though, we were using non-augmentable baseplates with the original Palacos® R&G (Heraeus Medical, Hanau, Germany) cement. In that year, we found that there were 5 knees which went onto aseptic loosening. The rate of failure of the non-augmentable baseplate did not increase further after the cement was changed although because the overall numbers per year were very small, statistical analysis has not been performed. Previous evidence from the Norwegian arthroplasty register has shown similar survival of TKAs cemented with Palacos versus those cemented with Refobacin [27].
To our knowledge, ours is the first study to compare aseptic loosening rates between these two baseplate types although a recent paper compared rates of aseptic loosening between the NexGen non-modular baseplate and Sigma® P.F.C® TKA (DePuy Synthes, Warsaw, Indiana) and found much higher rates of aseptic loosening of the NexGen tibial baseplate [24]. Whilst our rates of aseptic loosening from both implants are likely to be within what might be considered acceptable limits, the difference observed highlights the effect that minor changes to an implant can have on outcomes in the medium term. With a great deal of heterogeneity within arthroplasty registry data, it is important that clinicians remain alert to seemingly minor implant changes within their own units and publish their own results when important trends are observed. Our regional Zimmer Biomet representative has confirmed that in the last ten years, 78.84% of all primary NexGen knees implanted in the UK were non-augmentable.
In conclusion, we have demonstrated a significantly higher rate of aseptic loosening in the Zimmer NexGen TKA when using a non-augmentable 7° baseplate compared to an augmentable 7° baseplate. Further investigation of the rates of aseptic loosening in the non-augmentable baseplate at other units may be warranted to determine if these findings are replicable or if they may in part be due to the introduction of Refobacin® cement. Given the lower cost of the non-augmentable baseplate, a cost analysis based on the increased rate of revision observed may also be useful in order to consider whether there is any financial benefit in using this implant.
Data and materials availability
Not applicable.
References
Ethgen O, Bruyère O, Richy F et al (2004) Health-related quality of life in total hip and total knee arthroplasty: a qualitative and systematic review of the literature. JBJS 86:963–974
National Joint Registry 17th Annual report accessed at https://reports.njrcentre.org.uk/. Accessed 25 Nov 2020
Patel A, Pavlou G, Mújica-Mota RE, Toms AD (2015) The epidemiology of revision total knee and hip arthroplasty in England and Wales. Bone Jt J 97-B:1076–1081. https://doi.org/10.1302/0301-620X.97B8.35170
Schroer WC, Berend KR, Lombardi AV et al (2013) Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty 28:116–119. https://doi.org/10.1016/j.arth.2013.04.056
Arsoy D, Pagnano MW, Lewallen DG et al (2013) Aseptic tibial debonding as a cause of early failure in a modern total knee arthroplasty design. Clin Orthop 471:94–101. https://doi.org/10.1007/s11999-012-2467-4
Keohane D, Power F, Cullen E et al (2020) High rate of tibial debonding and failure in a popular knee replacement: a cause for concern. Knee 27:459–468. https://doi.org/10.1016/j.knee.2019.10.001
Parvizi J, Tan TL, Goswami K et al (2018) The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 33:1309-1314.e2. https://doi.org/10.1016/j.arth.2018.02.078
Hampton CB, Berliner ZP, Nguyen JT, et al (2020) Aseptic loosening at the tibia in total knee arthroplasty: a function of cement mantle quality? J Arthroplasty S0883540320301893. https://doi.org/10.1016/j.arth.2020.02.028
Shi X, Shen B, Kang P et al (2013) The effect of posterior tibial slope on knee flexion in posterior-stabilized total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21:2696–2703. https://doi.org/10.1007/s00167-012-2058-7
Kang K-T, Koh Y-G, Son J et al (2018) Influence of increased posterior tibial slope in total knee arthroplasty on knee joint biomechanics: a computational simulation study. J Arthroplasty 33:572–579. https://doi.org/10.1016/j.arth.2017.09.025
Stambough JB, Edwards PK, Mannen EM et al (2019) Flexion instability after total knee arthroplasty. J Am Acad Orthop Surg 27:642–651. https://doi.org/10.5435/JAAOS-D-18-00347
Bini SA, Chen Y, Katod M, Paxton EW (2013) Does pre-coating total knee tibial implants affect the risk of aseptic revision? Bone Jt J 95-B:367–370. https://doi.org/10.1302/0301-620X.95B3.27585
Harris WH, Schiller AL, Scholler JM et al (1976) Extensive localized bone resorption in the femur following total hip replacement. J Bone Jt Surg 58:612–618
Howie DW, Haynes DR, Rogers SD et al (1993) The response to particulate debris. Orthop Clin North Am 24:571–581
Lombardi AV, Berasi CC, Berend KR (2007) Evolution of tibial fixation in total knee arthroplasty. J Arthroplasty 22:25–29. https://doi.org/10.1016/j.arth.2007.02.006
Schmalzried TP, Jasty M, Harris WH (1992) Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am 74:849–863
Sundfeldt M, Carlsson LV, Johansson CB et al (2006) Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop 77:177–197. https://doi.org/10.1080/17453670610045902
Quinn JM, Neale S, Fujikawa Y et al (1998) Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int 62:527–531. https://doi.org/10.1007/s002239900473
Jasty MJ, Floyd WE, Schiller AL et al (1986) Localized osteolysis in stable, non-septic total hip replacement. J Bone Joint Surg Am 68:912–919
Berend M, Ritter M, Meding J et al (2004) The Chetranjan Ranawat Award: tibial component failure mechanisms in total knee arthroplasty. Clin Orthop 428:26–34. https://doi.org/10.1097/01.blo.0000148578.22729.0e
Abdel MP, Bonadurer GF, Jennings MT, Hanssen AD (2015) Increased aseptic tibial failures in patients with a BMI ≥35 and well-aligned total knee arthroplasties. J Arthroplasty 30:2181–2184. https://doi.org/10.1016/j.arth.2015.06.057
Cherian JJ, Jauregui JJ, Banerjee S et al (2015) What host factors affect aseptic loosening after THA and TKA? Clin Orthop Relat Res 473:2700–2709. https://doi.org/10.1007/s11999-015-4220-2
Foran JRH, Whited BW, Sporer SM (2011) Early aseptic loosening with a precoated low-profile tibial component: a case series. J Arthroplasty 26:1445–1450. https://doi.org/10.1016/j.arth.2010.11.002
Fuchs R, Mills EL, Clarke HD et al (2006) A third-generation, posterior-stabilized knee prosthesis. early results after follow-up of 2 to 6 years. J Arthroplasty 21:821–825. https://doi.org/10.1016/j.arth.2005.10.008
Ip D, Wu WC, Tsang WL (2003) Early results of posterior-stabilised NexGen Legacy total knee arthroplasty. J Orthop Surg Hong Kong 11:38–42. https://doi.org/10.1177/230949900301100109
Scottish Arthroplasty Project report (2009) Accessed at https://www.arthro.scot.nhs.uk/docs/Scottish_Arthroplasty_Project_Report_2009.pdf at 1720 on 25 Nov 2020
Birkeland Ø, Espehaug B, Havelin LI, Furnes O (2017) Bone cement product and failure in total knee arthroplasty. Acta Orthop 88:75–81. https://doi.org/10.1080/17453674.2016.1256937
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to the article in its current form.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was sought from the regional ethics committee and was deemed unnecessary given that the study only involved retrospective analysis of a prospectively collated database.
Consent to participate
All patients gave informed consent to have their records included in the database.
Consent to publish
All patients who contributed to the data base consented to their data being used for purposes of publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Level of Evidence: Level III
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brown, M., Ramasubbu, R., Jenkinson, M. et al. Significant differences in rates of aseptic loosening between two variations of a popular total knee arthroplasty design. International Orthopaedics (SICOT) 45, 2859–2867 (2021). https://doi.org/10.1007/s00264-021-05151-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-021-05151-w