Abstract
Purpose
The optimal treatment after neoadjuvant chemoimmunotherapy for patients with stage III non-small cell lung cancer (NSCLC) is unclear. This study aimed at comparing the efficacy and safety of chemoradiotherapy and surgery after neoadjuvant chemoimmunotherapy in stage III NSCLC.
Materials and methods
We conducted a real-world multicenter retrospective study on patients with stage III NSCLC who received surgery or chemoradiotherapy after neoadjuvant chemoimmunotherapy between October 2018 and December 2022. Progression-free survival (PFS) and overall survival (OS) were assessed from the initiation of neoadjuvant treatment and estimated by the Kaplan‒Meier method. Univariate and multivariate Cox regression models were used to examine potential prognostic factors. One-to-one propensity score matching (PSM) was used to further minimize confounding.
Results
A total of 239 eligible patients were enrolled, with 104 (43.5%) receiving surgery and 135 (56.5%) receiving CRT. After 1:1 PSM, 1- and 2-year PFS rates in patients receiving radical surgery (rSurgery group) vs. patients receiving definitive cCRT (dCCRT group) were 80.0% vs. 79.2% and 67.2% vs. 53.1%, respectively (P = 0.774). One- and 2-year OS rates were 97.5% vs. 97.4% and 87.3% vs. 89.9%, respectively (P = 0.558). Patients in the dCCRT group had a numerically lower incidence of distant metastases compared to those in the rSurgery group (42.9% vs. 70.6%, P = 0.119). The incidence of treatment-related adverse events was similar in both groups, except that the incidence of grade 3/4 hematological toxicity was significantly higher in the dCCRT group (30.0% vs. 10.0%, P = 0.025).
Conclusion
Following neoadjuvant chemoimmunotherapy, definitive concurrent chemoradiotherapy may achieve noninferior outcomes to radical surgery in stage III NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stage III non-small cell lung cancer (NSCLC), which accounts for approximately one-third of NSCLC cases, represents a heterogeneous group for which optimal treatment is uncertain [1]. Based on the primary tumor extension and nodal involvement, stage III NSCLC is categorized into resectable, potentially resectable and unresectable disease [2]. Classically, the standard of care for patients with unresectable stage III NSCLC in the immunotherapy era is concurrent chemoradiotherapy (cCRT) followed by consolidation immunotherapy (the PACIFIC regimen) [3, 4]. However, the optimal sequence of immunotherapy and radiotherapy remains unclear, and upfront immunotherapy before chemoradiotherapy (CRT) is increasingly recommended to downsize tumor and increase the likelihood of success with subsequent definitive CRT [5, 6]. For patients with resectable stage III disease, the combination of immunotherapy and surgery could provide tremendous potential benefits and has led to the increasing application of neoadjuvant immunotherapy [7,8,9]. However, the impressive efficacy of neoadjuvant immunotherapy leads to questions regarding the conversion of unresectable status to resectable status. Several studies have shown the promising outcomes of neoadjuvant chemoimmunotherapy followed by surgery in patients with unresectable stage III NSCLC [10,11,12], suggesting that post-conversion surgical resection may play a role in this scenario. Interestingly, with a deeper understanding of immunotherapy, robust evidence has shown that the function and integrity of tumor-draining lymph nodes (dLNs) appear to correlate with the efficacy of immunotherapy [13,14,15]. Compared with radical surgery involving regional lymphadenectomy, chemoradiotherapy, while also impairing the dLNs, appears to preserve the function and integrity of dLNs better with the help of some predictive models, such as estimated radiation doses to immune cells (EDIC) [16, 17], which in turn may lead to a noninferior outcome to surgery. Considering that previous studies in the preimmunotherapy era failed to demonstrate superior PFS and OS in patients with stage IIIA (N2) and selected IIIB who received surgery after neoadjuvant chemotherapy compared to CRT [18, 19], we therefore conducted this real-world multicenter retrospective study to investigate the optimal treatment after neoadjuvant chemoimmunotherapy.
Materials and methods
Patient selection
Patients with stage III NSCLC who received surgery or CRT after neoadjuvant chemoimmunotherapy at Tianjin Medical University Cancer Institute & Hospital, The Fourth Hospital of Hebei Medical University and Tianjin Cancer Hospital Airport Hospital between October 2018 and December 2022 were included in this multicenter, retrospective study. The inclusion criteria were as follows: (1) age ≥ 18, (2) histopathology-proven stage III NSCLC and (3) treatment with neoadjuvant chemoimmunotherapy followed by surgery or CRT. The exclusion criteria included (1) patients with mutant driver genes, such as epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements, (2) history of any cancer-specific treatment, (3) tumor progression before immunotherapy and (4) immunotherapy concurrent with radiotherapy (RT) or as part of a clinical trial.
All patients were assessed by a multidisciplinary team (MDT), including thoracic surgeons, radiation oncologists, radiologists, etc., before treatment and after induction therapy, with further consideration of patient treatment preferences to determine the subsequent treatment modality. In general, our MDT team prefers surgery for patients with more localized lesions and single-station lymph node metastases, while CRT for patients with multiple-station or bulky N2. According to the treatment modality after chemoimmunotherapy, the cohort of patients was divided into a surgery group and a CRT group. The patients’ baseline demographic and therapeutic data were extracted from their medical records. Individual NSCLC cases’ histological type and stage were determined according to the WHO criteria [20] and the International Association for the Study of Lung Cancer classification (8th edition) [21], respectively. The age-adjusted Charlson comorbidity index (aCCI) was used to assess the comorbidities between groups [22]. The cutoff point was determined by the median score.
This study conformed to the provisions of the Declaration of Helsinki (as revised in 2013) and was approved by the institutional medical ethics committee (No. bc2023063).
Drug treatment
The PD-1/PD-L1 inhibitors used including atezolizumab, camrelizumab, durvalumab, nivolumab, pembrolizumab, penpulimab, sintilimab, tislelizumab or toripalimab. These nine kinds of immune checkpoint inhibitors (ICIs) have been approved for the treatment of NSCLC based on the promising outcomes in NSCLC patients [4, 7, 23,24,25,26,27,28,29]. Different chemotherapy regimens for patients were adopted depending on the histological type of the tumor, the individual clinical condition of the patient, etc.
Study outcomes
Clinical outcomes, including PFS and OS, were assessed. PFS was estimated from the start of neoadjuvant treatment to the date of first documented event of disease progression, death without progression or last follow-up. OS was calculated from the initiation of neoadjuvant treatment until death or last follow-up. Patients were followed up every 3 months for the first 2 years after surgery or CRT and every 6 months thereafter. Individual patients’ treatment-related adverse events (TRAEs) were evaluated according to CTCAE version 5.0. In the exploratory analysis, in order to better compare with the PACIFIC trial, we further calculated PFS and OS from the end of CRT in patients who completed CRT without PD.
Statistical analysis
Patient characteristics were analyzed between treatment groups using the Chi-square test or Fisher’s exact test for categorical variables. The Kaplan‒Meier method was used to estimate PFS and OS, which were then evaluated using the log-rank test. When the univariate analysis yielded a P value of < 0.15, the variable was incorporated into the multivariate Cox regression analysis. One-to-one propensity score matching (PSM) with a caliper of 0.02 was performed to further minimize confounding. Propensity scores were calculated based on age, sex, WHO histology, stage, adjuvant ICI, ECOG performance status and aCCI. Subgroup analyses for PFS and OS were performed to assess the consistency of treatment effects in patient subgroups. Subgroup analyses used an unstratified Cox proportional hazards model with treatment as a covariate. P value inferior to 0.05 was considered statistically significant. ALL statistical analyses were performed using SPSS version 25 (IBM, Armonk, NY, USA).
Results
Baseline characteristics
A total of 239 consecutive eligible patients were included in this study. Among them, 104 (43.5%) received surgery and 135 (56.5%) received CRT. The median age was 63 years (range 27–78). More patients in the surgery group had an ECOG PS score of 0 and fewer comorbidities. Detailed clinical characteristics are shown in Table 1.
Treatment
All patents were treated with neoadjuvant chemoimmunotherapy and the immune checkpoint inhibitors (ICIs) included atezolizumab (0.4%, n = 1), camrelizumab (9.2%, n = 22), durvalumab (1.3%, n = 3), nivolumab (5.9%, n = 14), pembrolizumab (25.1%, n = 60), penpulimab (0.8%, n = 2), sintilimab (37.2%, n = 89), tislelizumab (18.4%, n = 44) and toripalimab (1.7%, n = 4).
In the surgery group, after receiving a median of 3 cycles of neoadjuvant immunotherapy (range 1–6), all patients received sleeve resection, lobectomy or pneumonectomy combined with routine mediastinal lymphadenectomy, except 2 patients received open thoracotomy. Three patients in the surgery group did not undergo complete resection, while the remaining 101 patients had R0 resection status. One of these three patients subsequently received postoperative radiotherapy, one received subsequent chemoimmunotherapy and one discontinued treatment. Forty-eight (46.2%) patients further received a median of 4 cycles of adjuvant immunotherapy (range 1–24), and 6 (5.8%) patients received postoperative radiotherapy. The type of ICI used for adjuvant immunotherapy was the same as that used preoperatively, with only one patient using the PD-L1 inhibitor and the remaining 47 patients using the PD-1 inhibitor. Among the 48 patients, 14 (29.2%) completed 1 year of adjuvant immunotherapy, and 34 discontinued due to disease progression (22.9%, n = 11), adverse events (10.4%, n = 5), economic burden (18.8%, n = 9) and patient request (18.8%, n = 9).
In the CRT group, after receiving a median of 4 cycles of neoadjuvant immunotherapy (range 1–12), 90 patients received sequential CRT (sCRT), while the remaining 45 patients received cCRT. All patients received a median dose of 60 Gy (range 45–66.0) of radiotherapy, and the majority of chemotherapy regimens were platinum-based doublet chemotherapy (95.6%, n = 129). Forty-nine (36.3%) patients further received adjuvant immunotherapy. Similarly, the type of ICI used for adjuvant immunotherapy after CRT was consistent with that used before CRT, with only two patients using the PD-L1 inhibitor and the remaining 47 patients using the PD-1 inhibitor. Among them, 15 (30.6%) completed 1 year of adjuvant immunotherapy, 34 discontinued due to disease progression (26.5%, n = 13), adverse events (8.2%, n = 4), economic burden (16.3%, n = 8), patient request (12.2%, n = 6) and 3 patients are still under treatment (6.1%).
Efficacy and safety
In the whole population, the median follow-up from the initiation of neoadjuvant treatment was 26.0 months (range 3.7–60.9 months). Median PFS was 26.1 months, and median OS was not reached (NR). As data cutoff, 115 patients had progressive disease (PD), with 45 (43.3%) in the surgery group and 70 (51.9%) in the CRT group. Of these 115 patients who progressed, one in the surgery group and four in the CRT group progressed during neoadjuvant chemoimmunotherapy. The patient in the surgery group underwent open thoracotomy followed by chemoimmunotherapy, and three of the four patients in the CRT group received sCRT instead of planned surgery. Fifty patients had died at the time of analysis, including 17 (16.3%) in the surgery group and 33 (24.4%) in the CRT group. All patients died of lung cancer, except one patient in the surgery group who died of postoperative pulmonary embolism and four patients in the CRT group who died of other causes. Three of these four patients in the CRT group died of COVID-19 infection, heart disease and accident, respectively, and none of them had tumor progression, while the other patient died of COVID-19 infection after locoregional progression.
The median PFS, 1- and 2-year PFS rates were NR, 75.0% and 61.4% in the surgery group, and 23.2 months, 76.7% and 45.1% in the CRT group. While the median OS, 1- and 2-year OS rates were NR, 96.2% and 89.9% in the surgery group, and 46.0 months, 92.5% and 78.8% in the CRT group. In the exploratory analysis, median PFS and OS from the end of CRT for patients who completed CRT without PD were 17.7 and 40.6 months, respectively. One- and 2-year PFS rates were 64.3% and 40.7%, respectively, while 1- and 2-year OS rates were 88.0% and 78.2%, respectively.
We further compared the incidence of common TRAEs between the two groups. The incidence of TRAEs was similar in both groups, except that the incidence of grade 3/4 hematological toxicity was significantly higher in the CRT group (Table 2). Three patients (2.9%) in the surgery group developed esophagitis following postoperative radiotherapy. Of note, one patient developed a postoperative pulmonary embolism and 38.5% (40/104) of patients in the surgery group developed postoperative pneumonia, which resolved after antibiotic therapy.
Radical surgery vs. definitive cCRT
We further investigated the prognostic impact of different radical treatments after neoadjuvant chemoimmunotherapy. After excluding patients with disease progression during neoadjuvant chemoimmunotherapy and restricting to those who underwent radical surgery/definitive cCRT, a total of 143 patients were included, of whom 101 underwent radical surgery (rSurgery group) and 42 underwent definitive cCRT (dCCRT group). Baseline characteristics of both patient groups are detailed in Table 3.
Median PFS was NR in either group, with a 1-year PFS rate of 77.2% in the rSurgery group vs. 80.2% in the dCCRT group and a 2-year PFS rate of 63.2% vs. 53.0% (P = 0.952, Fig. 1A). Median OS were all NR, with a 1-year OS rate of 96.0% vs. 97.5% and a 2-year OS rate of 89.6% vs. 87.1% (P = 0.924, Fig. 1B). Univariate and multivariate analyses further confirmed that the choice of radical treatment after neoadjuvant chemoimmunotherapy was not an independent factor affecting PFS (HR = 0.872, P = 0.663, Table S1 in the supplementary material) and OS (HR = 0.917, P = 0.882, Table S2 in the supplementary material). Further subgroup analyses showed no advantage in PFS or OS favoring either radical surgery or definitive cCRT after neoadjuvant chemoimmunotherapy across patient demographic characteristics and baseline clinicopathological features (Fig. 2).
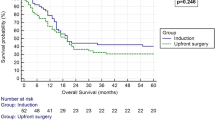
PFS and OS between the rSurgery and dCCRT groups before and after PSM. a PFS from the initiation of neoadjuvant treatment before PSM. b OS from the initiation of neoadjuvant treatment before PSM. c PFS from the initiation of neoadjuvant treatment after PSM. d OS from the initiation of neoadjuvant treatment after PSM. PFS progression-free survival, OS overall survival, dCCRT definitive concurrent chemoradiotherapy; rSurgery, radical surgery, PSM propensity score matching
Subgroup analyses of prognostic factors for PFS and OS in patients receiving radical treatment. PFS progression-free survival; OS, overall survival, dCCRT definitive concurrent chemoradiotherapy; rSurgery, radical surgery, NOS not otherwise specified, ICI immune checkpoint inhibitor, ECOG Eastern Cooperative Oncology Group, aCCI age-adjusted Charlson comorbidity index
After a further 1:1 PSM, baseline characteristics between the two treatment groups were basically balanced (Table 3). Still, there was no significant difference in PFS and OS between the rSurgery group and the dCCRT group. One- and 2-year PFS rates were 80.0% vs. 79.2% and 67.2% vs. 53.1%, respectively (P = 0.774, Fig. 1C). While 1- and 2-year OS rates were 97.5% vs. 97.4% and 87.3% vs. 89.9%, respectively (P = 0.558, Fig. 1D).
Regarding the pattern of relapse, there were three patients in the rSurgery group and one patient in the dCCRT group had unclear patterns of relapse. After removing patients with unknown patterns of relapse, there was no significant difference between the two treatment groups before PSM. While in the matched population, patients in the dCCRT group showed a numerically lower incidence of distant metastases compared to those in the rSurgery group (42.9% vs. 70.6%, P = 0.119, Table 4).
In terms of the incidence of TRAEs, the incidence of grade 3/4 hematological toxicity remained higher in the dCCRT group compared to the rSurgery group in the matched population (30.0% vs. 10.0%, P = 0.025, Table 5), while the incidence of other TRAEs was not significantly different between the two groups.
Discussion
The findings of this real-world study demonstrate comparable outcomes between definitive concurrent chemoradiotherapy and radical surgery following neoadjuvant chemoimmunotherapy in stage III NSCLC. These results are very interesting and should be considered hypothesis generating for future evaluation of these treatment modalities as neoadjuvant chemoimmunotherapy is increasingly used in clinical practice for stage III NSCLC, but evidence on the optimal treatment after neoadjuvant chemoimmunotherapy remains limited. To the best of our knowledge, this is the first study to directly compare the efficacy and safety of surgery with chemoradiotherapy after neoadjuvant chemoimmunotherapy through real-world data.
Debate over optimal treatment for stage III NSCLC does not appear to be diminishing with the advent of immunotherapy. Whether patients are operable or inoperable, immunotherapy in combination with surgery or CRT can significantly improve survival and has become the dominant treatment modality for stage III NSCLC [3, 7, 9, 30]. Although several decision-making surveys in stage III NSCLC have shown that surgery is the preferred option for patients with non-bulky mediastinal lymph node involvement or single-station N2, and CRT is the preferred option for patients with more extensive lymph node involvement [31,32,33], the impressive efficacy of neoadjuvant immunotherapy is gradually obscuring the already ill-defined concept of resectability and has led to its gradual application in potentially resectable and even unresectable NSCLC. Notably, a recent study highlighted the importance of dLNs in antitumor immune responses in humans [15], raising the question of whether there is a negative effect on antitumor immunity of regional lymphadenectomy in radical surgery or whether there is an option that can be an alternative to radical surgery involving regional lymphadenectomy in stage III NSCLC. In this study, the 2-year PFS rate of approximately 61% and the 2-year OS rate of approximately 89% of patients who underwent surgery after neoadjuvant chemoimmunotherapy in this study were similar to previous clinical trials [7, 9, 34]. And patients in the CRT group also showed comparable survival to those in the PACIFIC trial. Median PFS and 2-year PFS rate calculated from the end of CRT (17.7 months and 40.7%, respectively) were similar to those reported in the PACIFIC trial (16.9 months and 45.0%, respectively). Median OS and 2-year OS rate (40.6 months and 78.2%, respectively) were also noninferior to that from the PACIFIC trial (47.5 months and 66.3%, respectively) [4]. These survival data demonstrate comparable real-world efficacy of these two treatment modalities to the results of the clinical trials that established the current dominant treatment paradigm for stage III NSCLC.
Previous studies have demonstrated the superiority of cCRT to sCRT in patients with stage III NSCLC in terms of OS and reduced risk of locoregional progression, even after neoadjuvant chemoimmunotherapy [35,36,37]. However, to date, no phase III randomized trial has evaluated the application of immunotherapy in multimodal treatment regimens for patients with stage III NSCLC stratified by definitive treatment with surgery or CRT. Only a phase II trial conducted by Wu et al. found that surgery after neoadjuvant chemoimmunotherapy appeared to be superior to definitive cCRT (1-year EFS 74.4% vs. 55.9%), but they focused on a highly selected population stratified by PD-L1 expression and followed by an MDT to determine subsequent treatment [12]. Considering that patients receiving definitive cCRT in this study accounted for only 1/3 of patients receiving CRT, the application of definitive cCRT and radical surgery after neoadjuvant chemoimmunotherapy was further investigated. In accordance with the results in the preimmunotherapy era [18, 19], our results indicate that definitive cCRT was noninferior to radical surgery after neoadjuvant chemoimmunotherapy, even across all subgroups. The observed 1- and 2-year PFS rates in patients receiving definitive cCRT were 79.2% and 53.1%, respectively, while the 1- and 2-year OS rates were 97.4% and 89.9%, respectively, noninferior to the results of the AFT-16 (66% for 1-year PFS rate) and KEYNOTE-799 (approximately 60% for 2-year PFS rate and approximately 70% for 2-year OS rate) trials evaluating the safety and efficacy of immunotherapy before radiotherapy [23, 38].
Interestingly, patients receiving definitive cCRT demonstrated a similar incidence of locoregional recurrence but a numerically lower incidence of distant metastases compared to those receiving radical surgery, which is different from previous studies in the preimmunotherapy era [39, 40]. Previous studies showed that patients who received radiotherapy, even with stereotactic ablative radiotherapy (SABR), still had a higher incidence of locoregional recurrence, although the incidence of distant metastases appeared to have decreased compared to surgery [18, 39]. Of note, in operable stage I NSCLC patients who received SABR, the 3-year regional recurrence-free survival was 90% and the 3-year metastasis-free survival was 97% in Chang’s pooled study, yet the 2-year regional recurrence-free survival decreased to 53%, and the 2-year metastasis-free survival decreased to 76% in patients who received surgery after SABR [39, 41]. Combined with recent studies highlighting the importance of dLNs, we therefore speculate that the similar incidence of locoregional recurrence and relatively lower incidence of distant failure in our dCCRT group compared to the rSurgery group may be correlated with the addition of neoadjuvant chemoimmunotherapy and that definitive cCRT may preserve regional lymph nodes better than radical surgery and thus protect antitumor immunity. The 12-month incidence of locoregional recurrence and distant metastases in this study was 16.7% and 11.1%, respectively, which appears to be better than previous studies on the failure patterns of the PACIFIC regimen (approximately 20% for 12-month locoregional recurrence and approximately 30% for 12-month distant metastasis) [42,43,44]. Taken together, definitive cCRT may achieve noninferior outcomes to radical surgery after neoadjuvant chemoimmunotherapy with a numerically decreased incidence of distant failure.
Although the two different local treatment modalities resulted in a slightly different spectrum of TRAEs in the two treatment groups in this study, the common TRAEs were still relatively similar between the two treatment groups. There was no significant difference in the incidence of common TRAEs in either the whole population or the matched population, except for grade 3/4 hematological toxicity, which was higher but still acceptable in patients receiving CRT, suggesting that the safety of the two treatment modalities is relatively comparable. Regarding the incidence of pneumonia, which is of great concern, we note that although there was no significant difference in the incidence of pneumonia between the two groups, more than one-third of patients who underwent surgery developed postoperative pneumonia and were treated with antibiotics, and that the majority of patients received antibiotics in the perioperative period. However, there is increasing evidence that the application of antibiotics may reduce the efficacy of immunotherapy by disrupting the balance of gut microbiome [45,46,47]. It is unclear whether the perioperative application of antibiotics contributed to the similar outcomes of the two radical treatment modalities in this study, and large prospective clinical trials are needed to validate these findings in the future.
It is worth noting that although definitive cCRT following neoadjuvant chemoimmunotherapy appears to be noninferior to radical surgery in the present study, it must be acknowledged that the poor proportion of definitive cCRT also reflects to some extent the real-world treatment patterns of stage III NSCLC. A web-based survey of US oncologists found that an average of about 69% of patients with new unresectable stage III NSCLC receive radiotherapy, with only 48% receiving cCRT [48]. Moreover, in a prospective multicenter study investigating real-world treatment patterns for stage III NSCLC in China, only 41.3% (142/344) of patients with unresectable disease received CRT, and 62.7% (89/142) of these patients received cCRT. In contrast, all patients with resectable disease received surgery [49]. More importantly, patients who are inoperable or refuse surgery may not be able to tolerate definitive cCRT. Nevertheless, the key highlight of this study is the combination of immunotherapy, surgery and radiotherapy, which may break the barrier of the seemingly opposite sequence of immunotherapy application for resectable versus unresectable stage III NSCLC. Although several studies have shown the efficacy and safety of upfront immunotherapy before radiotherapy, the current standard of care for unresectable stage III NSCLC remains the PACIFIC regimen due to the absence of direct comparison with the PACIFIC regimen [23, 38, 50, 51]. However, recently published population-based study demonstrated better survival after surgery following neoadjuvant chemoimmunotherapy than cCRT followed by immunotherapy in patients with stage III-N2 NSCLC [52]. The comparable outcomes of the two radical treatment modalities after neoadjuvant chemoimmunotherapy in the present study suggest that patients with stage III NSCLC, whether resectable or unresectable, may benefit equally from several cycles of neoadjuvant chemoimmunotherapy followed by either radical treatment. The addition of neoadjuvant chemoimmunotherapy could prolong survival in patients with resectable NSCLC, while in patients with unresectable NSCLC, it could downsize initial tumors and contribute to following definitive CRT [7,8,9, 50], and those who receive definitive cCRT may achieve noninferior outcomes to those who receive radical surgery. Moreover, combining with adjuvant immunotherapy may further improve the outcome.
It should be pointed out that the present study has several limitations. First, this is a real-world retrospective study, and the inherent drawbacks of retrospective studies made some selection bias inevitable, while the lack of original imaging data in a proportion of patients made it difficult to further explore the prognostic impact of the two treatment modalities based on different N2 status, such as bulky or multi-station N2. In addition, the moderate sample size of patients receiving definitive cCRT limited the ability to draw a definitive conclusion, but our study provided the preliminary evidence for future large-scale and prospective trials. Furthermore, data regarding PD-L1 expression were sparse because it is not routinely tested in stage III NSCLC at our three centers. Meanwhile, this study did not further differentiate between the efficacy of various ICIs. Nevertheless, this multicenter study reflects to some extent the use and benefits of immunotherapy in stage III NSCLC in the real-world practice. Future studies should use the same ICI agent and stratify by PD-L1 expression to minimize confounding. Despite these limitations, this is the first study to investigate different treatment modalities after neoadjuvant chemoimmunotherapy in stage III NSCLC through real-world data. We believe this study may provide a new direction for research or an option for stage III NSCLC patients who refuse or cannot receive surgery after neoadjuvant chemoimmunotherapy.
Conclusions
Following neoadjuvant chemoimmunotherapy, definitive concurrent chemoradiotherapy may achieve noninferior outcomes to radical surgery. Prospective randomized trials are warranted to further validate these findings.
Data availability statement
Data will be made available on request.
References
Govindan R, Bogart J, Vokes EE (2008) Locally advanced non-small cell lung cancer: the past, present, and future. J Thorac Oncol 3(8):917–928. https://doi.org/10.1097/JTO.0b013e318180270b
Tan WL, Chua KLM, Lin CC et al (2020) Asian thoracic oncology research group expert consensus statement on optimal management of stage III NSCLC. J Thorac Oncol 15(3):324–343. https://doi.org/10.1016/j.jtho.2019.10.022
Antonia SJ, Villegas A, Daniel D et al (2017) Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377(20):1919–1929. https://doi.org/10.1056/NEJMoa1709937
Spigel DR, Faivre-Finn C, Gray JE et al (2022) Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol 40(12):1301–1311. https://doi.org/10.1200/JCO.21.01308
Lang P, Palma D (2021) Too big to fail: miracle drugs or false hope? Int J Radiat Oncol 110(2):264. https://doi.org/10.1016/j.ijrobp.2020.10.013
Chang JY (2021) When constrained by constraints: thinking outside of the box in both technology and biology. Int J Radiat Oncol 110(2):266–267. https://doi.org/10.1016/j.ijrobp.2020.10.014
Forde PM, Spicer J, Lu S et al (2022) Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. New Engl J Med 386(21):1973–1985. https://doi.org/10.1056/NEJMoa2202170
Rothschild SI, Zippelius A, Eboulet EI et al (2021) Swiss Group for Clinical Cancer Research (SAKK). SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer—a multicenter single-arm phase II trial. J Clin Oncol 39(26):2872–2880. https://doi.org/10.1200/JCO.21.00276
Wakelee H, Liberman M, Kato T et al (2023) Perioperative pembrolizumab for early-stage non–small-cell lung cancer. New Engl J Med 389(6):491–503. https://doi.org/10.1056/NEJMoa2302983
Deng H, Liu J, Cai X et al (2022) Radical minimally invasive surgery after immuno-chemotherapy in initially-unresectable stage IIIB non-small cell lung cancer. Ann Surg 275(3):e600–e602. https://doi.org/10.1097/SLA.0000000000005233
Xu H, Wang W, Yin J et al (2022) Efficacy and Safety of the PD-1 inhibitor combined with albumin-bound paclitaxel and nedaplatin in preoperative neoadjuvant therapy of unresectable stage III lung squamous cell carcinoma. Drug Des Devel Ther 16:4269–4277. https://doi.org/10.2147/DDDT.S388777
Wu Y, Zhou Q, Pan Y et al (2022) A phase 2 study of neoadjuvant SHR-1701 with or without chemotherapy (chemo) followed by surgery or radiotherapy (RT) in stage III unresectable NSCLC (uNSCLC). Ann Oncol 16(1):100104. https://doi.org/10.1016/j.iotech.2022.100361
Fear VS, Forbes CA, Neeve SA et al (2021) Tumour draining lymph node-generated CD8 T cells play a role in controlling lung metastases after a primary tumour is removed but not when adjuvant immunotherapy is used. Cancer Immunol Immun 70(11):3249–3258. https://doi.org/10.1007/s00262-021-02934-3
Spitzer MH, Carmi Y, Reticker-Flynn NE et al (2017) Systemic immunity is required for effective cancer immunotherapy. Cell 168(3):487-502.e15. https://doi.org/10.1016/j.cell.2016.12.022
Rahim MK, Okholm TLH, Jones KB et al (2023) Dynamic CD8+ T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell 186(6):1127–43.e18. https://doi.org/10.1016/j.cell.2023.02.021
Yin X, Luo J, Xu C et al (2021) Is a higher estimated dose of radiation to immune cells predictive of survival in patients with locally advanced non-small cell lung cancer treated with thoracic radiotherapy? Radiother Oncol 159:218–223. https://doi.org/10.1016/j.radonc.2021.03.026
McCall NS, McGinnis HS, Janopaul-Naylor JR et al (2022) Impact of radiation dose to the immune cells in unresectable or stage III non-small cell lung cancer in the durvalumab era. Radiother Oncol 174:133–140. https://doi.org/10.1016/j.radonc.2022.07.015
van Meerbeeck JP, Kramer GW, Van Schil PE et al (2007) Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. Jnci-J Natl Cancer I 99(6):442–450. https://doi.org/10.1093/jnci/djk093
Johnstone DW, Byhardt RW, Ettinger D, Scott CB (2002) Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol 54(2):365–369. https://doi.org/10.1016/s0360-3016(02)02943-7
Travis WD, Brambilla E, Nicholson AG et al (2015) The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10(9):1243–1260. https://doi.org/10.1097/JTO.0000000000000630
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT (2017) The eighth edition lung cancer stage classification. Chest 151(1): 193–203. Doi: https://doi.org/10.1016/j.chest.2016.10.010
Yang CC, Fong Y, Lin LC et al (2018) The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardio-thorac 53(1):235–240. https://doi.org/10.1093/ejcts/ezx215
Jabbour SK, Lee KH, Frost N et al (2021) Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol 7(9):1–9. https://doi.org/10.1001/jamaoncol.2021.2301
Han B, Jiao S, Chen J et al (2022) Final analysis of AK105-302: a randomized, double-blind, placebo-controlled, phase III trial of penpulimab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC. Immunooncol Technol 16:100164. https://doi.org/10.1016/j.iotech.2022.100164
Zhou C, Chen G, Huang Y et al (2021) Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Resp Med 9(3):305–314. https://doi.org/10.1016/s2213-2600(20)30365-9
Yang Y, Wang Z, Fang J et al (2020) Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a Randomized, double-blind, phase 3 study (oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol 15(10):1636–1646. https://doi.org/10.1016/j.jtho.2020.07.014
Wang J, Lu S, Yu X et al (2021) Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol 7(5):709–717. https://doi.org/10.1001/jamaoncol.2021.0366
Lu S, Wu L, Zhang W et al (2023) Perioperative toripalimab + platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer (NSCLC): interim event-free survival (EFS) analysis of the phase III Neotorch study. J Clin Oncol 41(36_suppl):425126. https://doi.org/10.1200/JCO.2023.41.36_suppl.425126
Spigel D, De Marinis F, Giaccone G et al (2019) IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Ann Oncol 30:v915. https://doi.org/10.1093/annonc/mdz293
Zhou Q, Chen M, Jiang O et al (2022) Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 23(2):209–219. https://doi.org/10.1016/S1470-2045(21)00630-6
Evison M, Edwards J, McDonald F, Popat S (2020) Stage III non-small cell lung cancer: a UK national survey of practice. Clin Oncol-UK 32(8):527–536. https://doi.org/10.1016/j.clon.2020.03.001
Putora PM, Leskow P, McDonald F et al (2020) International guidelines on stage III N2 nonsmall cell lung cancer: surgery or radiotherapy? ERJ Open Res. https://doi.org/10.1183/23120541.00159-2019
Glatzer M, Leskow P, Caparrotti, et al (2021) Stage III N2 non-small cell lung cancer treatment: decision-making among surgeons and radiation oncologists. Transl Lung Cancer R 10(4):1960–1968. https://doi.org/10.21037/tlcr-20-1210
Provencio M, Nadal E, González-Larriba JL et al (2023) Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. New Engl J Med 389(6):504–513. https://doi.org/10.1056/NEJMoa2215530
Aupérin A, Le Péchoux C, Rolland E et al (2010) Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 28(13):2181–2190. https://doi.org/10.1200/JCO.2009.26.2543
Curran WJ, Paulus R, Langer CJ et al (2011) Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. JNCI-Jnatl Cancer I 103(19):1452–1460. https://doi.org/10.1093/jnci/djr325
Guan S, Ren K, Zhang X et al (2023) Concurrent chemoradiotherapy versus radiotherapy alone after induction chemoimmunotherapy for stage III NSCLC patients who did not undergo surgery: a single institution retrospective study. Radiat Oncol 18(1):122. https://doi.org/10.1186/s13014-023-02305-5
Ross HJ, Kozono DE, Urbanic JJ et al (2021) AFT-16: Phase II trial of neoadjuvant and adjuvant atezolizumab and chemoradiation (CRT) for stage III non-small cell lung cancer (NSCLC). J Clin Oncol 39(15_suppl):8513–8513. https://doi.org/10.1200/JCO.2021.39.15_suppl.8513
Bosch-Barrera J, García-Franco C, Guillén-Grima F et al (2012) The multimodal management of locally advanced N2 non-small cell lung cancer: is there a role for surgical resection? A single institution’s experience. Clin Transl Oncol 14(11):835–841. https://doi.org/10.1007/s12094-012-0874-3
Chang JY, Senan S, Paul MA et al (2015) Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 16(6):630–637. https://doi.org/10.1016/S1470-2045(15)70168-3
Palma DA, Nguyen TK, Louie AV et al (2019) Measuring the integration of stereotactic ablative radiotherapy plus surgery for early-stage non-small cell lung cancer: a phase 2 clinical trial. JAMA Oncol 5(5):681–688. https://doi.org/10.1001/jamaoncol.2018.6993
Abe T, Saito S, Iino M et al (2021) Effect of durvalumab on local control after concurrent chemoradiotherapy for locally advanced non-small cell lung cancer in comparison with chemoradiotherapy alone. Thorac Cancer 12(2):245–250. https://doi.org/10.1111/1759-7714.13764
Offin M, Shaverdian N, Rimner A et al (2020) Clinical outcomes, local-regional control and the role for metastasis-directed therapies in stage III non-small cell lung cancers treated with chemoradiation and durvalumab. Radiother Oncol 149:205–211. https://doi.org/10.1016/j.radonc.2020.04.047
Bruni A, Scotti V, Borghetti P et al (2021) A real-world, multicenter, observational retrospective study of durvalumab after concomitant or sequential chemoradiation for unresectable stage III non-small cell lung cancer. Front Oncol 11:744956. https://doi.org/10.3389/fonc.2021.744956
Routy B, Le Chatelier E, Derosa L et al (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359(6371):91–97. https://doi.org/10.1126/science.aan3706
Derosa L, Hellmann MD, Spaziano M et al (2018) Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 29(6):1437–1444. https://doi.org/10.1093/annonc/mdy103
Eng L, Sutradhar R, Niu Y et al (2023) Impact of antibiotic exposure before immune checkpoint inhibitor treatment on overall survival in older adults with cancer: a population-based study. J Clin Oncol 41(17):3122–3134. https://doi.org/10.1200/JCO.22.00074
Cotarla I, Boron ML, Cullen SL et al (2020) Treatment decision drivers in stage III non–small-cell lung cancer: outcomes of a web-based survey of oncologists in the United States. JCO Oncol Pract 16(10):e1232–e1242. https://doi.org/10.1200/JOP.19.00781
Xing L, Yu J, Zhao R et al (2023) Real-world treatment patterns in stage III NSCLC patients: Interim results of a prospective, multicenter, noninterventional study (MOOREA). J Thorac Oncol 18(4_suppl):S111–S112. https://doi.org/10.1016/S1556-0864(23)00380-5
Wang Y, Zhang T, Wang J et al (2023) Induction immune checkpoint inhibitors and chemotherapy before definitive chemoradiation therapy for patients with bulky unresectable stage III non-small-cell lung cancer. Int J Radiat Oncol 116(3):590–600. https://doi.org/10.1016/j.ijrobp.2022.12.042
Guan S, Zhang S, Ren K et al (2023) Induction chemoimmunotherapy may improve outcomes of chemoradiotherapy in patients with unresectable stage III NSCLC. Front Immunol 14:1289207. https://doi.org/10.3389/fimmu.2023.1289207
Kumar A, Srinivasan D, Potter AL et al (2023) Induction chemoimmunotherapy with surgery versus concurrent chemoradiation followed by immunotherapy for stage III-N2 non-small cell lung cancer. J Thorac Cardiov Sur. https://doi.org/10.1016/j.jtcvs.2023.09.029
Funding
This research was funded by the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A).
Author information
Authors and Affiliations
Contributions
SG designed and conceived the study, wrote the manuscript. JS, YW and SH performed data curation. CC, DY and YH performed data analysis. KR performed data interpretation. JW and JW edited the manuscript. LZ designed and conceived the study, edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
This study has been specifically approved by the institutional medical ethics committee, and the ethics approval number is bc2023063. The requirement for informed consent was waived because of the retrospective nature of the research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Song Guan, Jifeng Sun and Yuan Wang contributed equally to this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guan, S., Sun, J., Wang, Y. et al. Chemoradiotherapy versus surgery after neoadjuvant chemoimmunotherapy in patients with stage III NSCLC: a real-world multicenter retrospective study. Cancer Immunol Immunother 73, 120 (2024). https://doi.org/10.1007/s00262-024-03696-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-024-03696-4