Abstract
Background
Concomitant medications may potentially affect the outcome of cancer patients. In this sub-analysis of the ARON-2 real-world study (NCT05290038), we aimed to assess the impact of concomitant use of proton pump inhibitors (PPI), statins, or metformin on outcome of patients with metastatic urothelial cancer (mUC) receiving second-line pembrolizumab.
Methods
We collected data from the hospital medical records of patients with mUC treated with pembrolizumab as second-line therapy at 87 institutions from 22 countries. Patients were assessed for overall survival (OS), progression-free survival (PFS), and overall response rate. We carried out a survival analysis by a Cox regression model.
Results
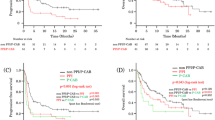
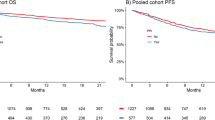
A total of 802 patients were eligible for this retrospective study; the median follow-up time was 15.3 months. PPI users compared to non-users showed inferior PFS (4.5 vs. 7.2 months, p = 0.002) and OS (8.7 vs. 14.1 months, p < 0.001). Concomitant PPI use remained a significant predictor of PFS and OS after multivariate Cox analysis. The use of statins or metformin was not associated with response or survival.
Conclusions
Our study results suggest a significant prognostic impact of concomitant PPI use in mUC patients receiving pembrolizumab in the real-world context. The mechanism of this interaction warrants further elucidation.







Similar content being viewed by others
References
Taguchi S, Kawai T, Nakagawa T, Miyakawa J, Kishitani K, Sugimoto K et al (2022) Improved survival in real-world patients with advanced urothelial carcinoma: a multicenter propensity score-matched cohort study comparing a period before the introduction of pembrolizumab (2003–2011) and a more recent period (2016–2020). Int J Urol 29(12):1462–1469. https://doi.org/10.1111/iju.15014
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L et al (2017) KEYNOTE-045 investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376(11):1015–1026. https://doi.org/10.1056/NEJMoa1613683
Peters S, Dziadziuszko R, Morabito A, Felip E, Gadgeel SM, Cheema P et al (2018) Atezolizumab versus chemotherapy in patients with platinum treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 28(9):1831–1839. https://doi.org/10.1038/s41591-022-01933-w
Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedkeet J et al (2017) Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicenter, single-arm, phase 2 trial. Lancet Oncol 18(3):312–322. https://doi.org/10.1016/S1470-2045(17)30065-7
Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumailyet R et al (2018) Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 19(1):51–64. https://doi.org/10.1016/S1470-2045(17)30900-2
Buti S, Bersanelli M, Perrone F, Tiseo M, Tucci M, Adamo V et al (2021) Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer 142:18–28. https://doi.org/10.1016/j.ejca.2020.09.033
Buti S, Bersanelli M, Perrone F, Bracarda S, Di Maio M, Giusti R et al (2021) Predictive ability of a drug-based score in patients with advanced non-small-cell lung cancer receiving first-line immunotherapy. Eur J Cancer 150:224–231. https://doi.org/10.1016/j.ejca.2021.03.041
Takada K, Buti S, Bersanelli M, Shimokawa M, Takamori S, Matsubara T et al (2022) Antibiotic-dependent effect of probiotics in patients with non-small cell lung cancer treated with PD-1 checkpoint blockade. Eur J Cancer 172:199–208. https://doi.org/10.1016/j.ejca.2022.06.002
Hopkins AM, Kichenadasse G, Karapetis CS, Rowland A, Sorich MJ (2020) Concomitant antibiotic use and survival in urothelial carcinoma treated with atezolizumab. Eur Urol 78(4):540–543. https://doi.org/10.1016/j.eururo.2020.06.061
Hopkins AM, Kichenadasse G, Karapetis CS, Rowland A, Sorich MJ (2020) Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin Cancer Res 26:5487–5493
Ruiz-BañobreJ M-DA, Fernández-Calvo O, Fernández-Núñez N, Medina-Colmenero A, Santomé L et al (2021) Rethinking prognostic factors in locally advanced or metastatic urothelial carcinoma in the immune checkpoint blockade era: a multicenter retrospective study. ESMO Open 6(2):100090. https://doi.org/10.1016/j.esmoop.2021.100090
Ishiyama Y, Kondo T, Nemoto Y, Kobari Y, Ishihara H, Tachibana H et al (2021) Antibiotic use and survival of patients receiving pembrolizumab for chemotherapy-resistant metastatic urothelial carcinoma. Urol Oncol 39(12):834.e21-834.e28. https://doi.org/10.1016/j.urolonc.2021.05.033
Fukuokaya W, Kimura T, Komura K, Uchimoto T, Nishimura K, Yanagisawa T et al (2022) Effectiveness of pembrolizumab in patients with urothelial carcinoma receiving proton pump inhibitors. Urol Oncol 40(7):346.e1-346.e8. https://doi.org/10.1016/j.urolonc.2022.02.020
Tomisaki I, Harada M, Minato A, Nagata Y, Kimuro R, Higashijima K et al (2022) Impact of the use of proton pump inhibitors on pembrolizumab effectiveness for advanced urothelial carcinoma. Anticancer Res 42(3):1629–1634. https://doi.org/10.21873/anticanres.15638
Kunimitsu Y, Morio K, Hirata S, Yamamoto K, Yamamoto K, Omura T, Hara T et al (2022) Effects of proton pump inhibitors on survival outcomes in patients with metastatic or unresectable urothelial carcinoma treated with pembrolizumab. Biol Pharm Bull 45(5):590–595. https://doi.org/10.1248/bpb.b21-00939
Okuyama Y, Hatakeyama S, Numakura K, Narita T, Tanaka T, Miura Y et al (2021) Prognostic impact of proton pump inhibitors for immunotherapy in advanced urothelial carcinoma. BJUI Compass 3:154–161. https://doi.org/10.1002/bco2.118
Rizzo A, Santoni M, Mollica V, Ricci AD, Calabrò C, Cusmai A et al (2022) The impact of concomitant proton pump inhibitors on immunotherapy efficacy among patients with urothelial carcinoma: a meta-analysis. J Pers Med 12(5):842. https://doi.org/10.3390/jpm12050842
Triadafilopoulos G, Roorda AK, Akiyama J (2013) Indications and safety of proton pump inhibitor drug use in patients with cancer. Expert Opin Drug Saf 12(5):659–672. https://doi.org/10.1517/14740338.2013.797961
Chalabi M, Cardona A, Nagarkar DR, Scala AD, Gandara DR, Rittmeyer A et al (2020) Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol 31(4):525–531. https://doi.org/10.1016/j.annonc.2020.01.006
Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC et al (2016) Proton pump inhibitors alter the composition of the gut microbiota. Gut 65(5):749–756. https://doi.org/10.1136/gutjnl-2015-310861
Deng R, Zhang H, Li Y, Shi Y (2022) Effect of antacid use on immune checkpoint inhibitors in advanced solid cancer patients: a systematic review and meta-analysis. J Immunother 46(2):43–55. https://doi.org/10.1097/CJI.0000000000000442
Ciccarese C, Iacovelli R, Buti S, Primi F, Astore S, Massari F et al (2022) Concurrent nivolumab and metformin in diabetic cancer patients: is it safe and more active? Anticancer Res 42(3):1487–1493. https://doi.org/10.21873/anticanres.15620
Santoni M, Molina-Cerrillo J, Myint ZW, Massari F, Buchler T, Buti S et al (2022) Concomitant use of statins, metformin, or proton pump inhibitors in patients with advanced renal cell carcinoma treated with first-line combination therapies. Target Oncol 17(5):571–581. https://doi.org/10.1007/s11523-022-00907-9
Santoni M, Massari F, Matrana MR, Basso U, De Giorgi U, Aurilio G et al (2022) Statin use improves the efficacy of nivolumab in patients with advanced renal cell carcinoma. Eur J Cancer 172:191–198. https://doi.org/10.1016/j.ejca.2022.04.035
Perrone F, Minari R, Bersanelli M, Bordi P, Tiseo M, Favari E et al (2020) The prognostic role of high blood cholesterol in advanced cancer patients treated with immune checkpoint inhibitors. J Immunother 43(6):196–203. https://doi.org/10.1097/CJI.0000000000000321
Cantini L, Pecci F, Hurkmans DP, Belderbos RA, Lanese A, Copparoni C et al (2021) High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur J Cancer 144:41–48. https://doi.org/10.1016/j.ejca.2020.10.031
Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S et al (2016) RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer 62:132–137. https://doi.org/10.1016/j.ejca.2016.03.081
Mollica V, Rizzo A, Montironi R, Cheng L, Giunchi F, Schiavina R et al (2020) Current strategies and novel therapeutic approaches for metastatic urothelial carcinoma. Cancers (Basel) 12(6):1449. https://doi.org/10.3390/cancers12061449
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R et al (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359(6371):91–97. https://doi.org/10.1126/science.aan3706
Seto CT, Jeraldo P, Orenstein R, Chia N, DiBaise JK (2014) Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome 2:42. https://doi.org/10.1186/2049-2618-2-42
Clooney AG, Bernstein CN, Leslie WD, Vagianos K, Sargent M, Laserna-Mendieta EJ et al (2016) A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment Pharmacol Ther 43(9):974–984. https://doi.org/10.1111/apt.13568
Tsuda A, Suda W, Morita H, Takanashi K, Takagi A, Koga Y et al (2015) Influence of proton-pump inhibitors on the luminal microbiota in the gastrointestinal tract. Clin Transl Gastroenterol 6(6):e89. https://doi.org/10.1038/ctg.2015.20
Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L et al (2016) Proton pump inhibitors affect the gut microbiome. Gut 65(5):740–748. https://doi.org/10.1136/gutjnl-2015-310376
Thouvenin J, Martínez Chanzá N, Alhalabi O, Lang H, Tannir NM, Barthélémy P et al (2021) Efficacy of immune checkpoint inhibitors in upper tract urothelial carcinomas: current knowledge and future directions. Cancers (Basel) 13(17):4341. https://doi.org/10.3390/cancers13174341
Su X, Lu X, Bazal SK, Compérat E, Mouawad R, Yao H et al (2021) Comprehensive integrative profiling of upper tract urothelial carcinomas. Genome Biol 22(1):7. https://doi.org/10.1186/s13059-020-02230-w
Taguchi S, Kawai T, Buti S, Bersanelli M, Uemura Y, Kishitani K, et al (2023) Validation of a drug-based score in advanced urothelial carcinoma treated with pembrolizumab. https://doi.org/10.2217/imt-2023-0028.
Chan KK, Oza AM, Siu LL (2003) The statins as anticancer agents. Clin Cancer Res 9:10–19
Keyomarsi K, Sandoval L, Band V (2003) Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res 9:10–19
Wong WW, Dimitroulakos J, Minden MD, Penn LZ (2002) HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16:508–519
Ferro M, Marchioni M, Lucarelli G, Vartolomei MD, Soria F, Terracciano D et al (2021) Association of statin use and oncological outcomes in patients with first diagnosis of T1 high grade non-muscle invasive urothelial bladder cancer: results from a multicenter study. Minerva Urol Nephrol 73(6):796–802. https://doi.org/10.23736/S2724-6051.20.04076-X
Haimerl L, Strobach D, Mannell H, Stief CG, Buchner A, Karl A et al (2022) Retrospective evaluation of the impact of non-oncologic chronic drug therapy on the survival in patients with bladder cancer. Int J Clin Pharm 44(2):339–347. https://doi.org/10.1007/s11096-021-01343-x
Samuel SM, Varghese E, Kubatka P, Triggle CR, Büsselberg D et al (2019) Metformin: the answer to cancer in a flower? Current knowledge and future prospects of metformin as an anti-cancer agent in breast cancer. Biomolecules 9(12):846. https://doi.org/10.3390/biom9120846
Chen YC, Li H, Wang J (2020) Mechanisms of metformin inhibiting cancer invasion and migration. Am J Transl Res 12(9):4885–4901
Acknowledgements
The authors would like to thank all patients voluntarily taking part in the study.
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
Manuscript: Use of concomitant proton pump inhibitors, statins, or metformin in patients treated with pembrolizumab for advanced urothelial carcinoma: Data from the ARON-2 retrospective study. Fiala et al. O. Fiala: Investigation, Writing - Original Draft M. Santoni: Conceptualization, Methodology, Investigation, Formal analysis, Writing - Original Draft S. Buti: Conceptualization, Methodology, Investigation, Writing - Original Draft All authors: Investigation
Corresponding author
Ethics declarations
Conflict of interest
O. Fiala received honoraria from Roche, Janssen, GSK and Pfizer for consultations and lectures unrelated to this project. S. Buti received honoraria as speaker at scientific events and advisory role by BMS, Pfizer, MSD, Ipsen, AstraZeneca, Merck, all unrelated to this project. M. Santoni has received research support and honoraria from Janssen, Bristol Myers Squibb, Ipsen, MSD, Astellas and Bayer, all unrelated to this project. R. Kanesvaran has received fees for speaker bureau and advisory board activities from the following companies; Pfizer, MSD, BMS, Eisai, Ipsen, Johnson and Johnson, Merck, Amgen, Astellas and Bayer, all unrelated to this project. E. Grande has received honoraria for speaker engagements, advisory roles or funding of continuous medical education from Adacap, AMGEN, Angelini, Astellas, Astra Zeneca, Bayer, Blueprint, Bristol Myers Squibb, Caris Life Sciences, Celgene, Clovis-Oncology, Eisai, Eusa Pharma, Genetracer, Guardant Health, HRA-Pharma, IPSEN, ITM-Radiopharma, Janssen, Lexicon, Lilly, Merck KGaA, MSD, Nanostring Technologies, Natera, Novartis, ONCODNA (Biosequence), Palex, Pharmamar, Pierre Fabre, Pfizer, Roche, Sanofi-Genzyme, Servier, Taiho, and Thermo Fisher Scientific and has received research grants from Pfizer, Astra Zeneca, Astellas, and Lexicon Pharmaceuticals, all unrelated to this project. F. S. M. Monteiro has received research support from Janssen, Merck Sharp Dome and honoraria from Janssen, Ipsen, Bristol Myers Squibb and Merck Sharp Dome, all unrelated to this project. C. Porta has received honoraria from Angelini Pharma, Astra Zeneca, BMS, Eisai, General Electric, Ipsen and MSD and acted as a Protocol Steering Committee Member for BMS, Eisai and MSD, all unrelated to this project. Z. Myint has received research support from Merck unrelated to this project. J. Molina-Cerrillo declares consultant, advisory or speaker roles for IPSEN, Roche, Pfizer, Sanofi, Janssen, and BMS and has received research grants from Pfizer, IPSEN and Roche, all unrelated to this project. P. Giannatempo has received research support from Ipsen, Astra Zeneca, MSD and honoraria for speaker engagements, advisory roles from Astellas, MSD, Janssen, Pfizer, all unrelated to this project. E. T. Lam has received institutional research funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Bristol-Myers Squibb, Pfizer, and F. Hoffmann-La Roche Ltd. The other authors declare to have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fiala, O., Buti, S., Takeshita, H. et al. Use of concomitant proton pump inhibitors, statins or metformin in patients treated with pembrolizumab for metastatic urothelial carcinoma: data from the ARON-2 retrospective study. Cancer Immunol Immunother 72, 3665–3682 (2023). https://doi.org/10.1007/s00262-023-03518-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03518-z