Abstract
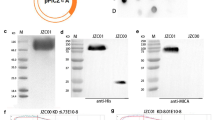
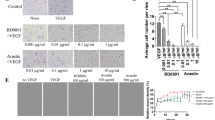
Antiangiogenic therapy has shown significant clinical benefits in gastric cancer (GC) and non-small cell lung cancer (NSCLC). However, their effectiveness is limited by the immunosuppressive tumor microenvironment. The MHC class I chain-related molecules A and B (MICA/B) are expressed in many human cancers, enabling elimination of cancer cells by cytotoxic lymphocytes through natural killer group 2D (NKG2D) receptor activation. To improve antiangiogenic therapy and prolong its efficacy, we generated a bi-specific fusion protein (mAb04-MICA). This was comprised of an antibody targeting VEGFR2 fused to a MICA α1–α2 ectodomain. mAb04-MICA inhibited proliferation of GC and NSCLC cells through specific binding to VEGFR2 and had superior anti-tumor efficacy in both GC and NSCLC-bearing mouse models compared with ramucirumab. Further investigation revealed that the mAb04-MICA promoted NKG2D+ NK cell activation and induced the tumor-associated macrophage (TAM) polarization from M2 type to M1 type both in vitro and in vivo. The polarization of TAMs upon NKG2D and MICA mediated activation has not yet been reported. Moreover, given the up-regulation of PD-L1 in tumors during anti-angiogenesis therapy, anti-PD-1 antibody enhanced the anti-tumoral activity of mAb04-MICA through stimulating infiltration and activation of NKs and CD8+T cells in responding tumors. Our findings demonstrate that dual targeting of angiogenesis and NKG2D, or in combination with the PD-1/PD-L1 blockade, is a promising anti-tumor therapeutic strategy. This is accomplished through maintaining or reinstating tumor immunosurveillance during treatment, which expands the repertoire of anti-angiogenesis-based cancer immunotherapies.








Similar content being viewed by others
References
Otrock ZK, Makarem JA, Shamseddine AI (2007) Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis 38(3):258–268
Shibuya M, Claesson-Welsh L (2006) Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 312(5):549–560
Xuan ZX, Li LN, Zhang Q, Xu CW, Yang DX, Yuan Y et al (2014) Fully human VEGFR2 monoclonal antibody BC001 attenuates tumor angiogenesis and inhibits tumor growth. Int J Oncol 45(6):2411–2420
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186
Ferrara N, Adamis AP (2016) Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov 15(6):385–403
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C et al (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383(9911):31–39
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15(11):1224–1235
Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T et al (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384(9944):665–673
Roodhart JM, Langenberg MH, Witteveen E, Voest EE (2008) The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol 3(2):132–143
Nandikolla AG, Rajdev L (2016) Targeting angiogenesis in gastrointestinal tumors: current challenges. Trans Gastroenterol Hepatol 1:67
Arnold D, Fuchs CS, Tabernero J, Ohtsu A, Zhu AX, Garon EB et al (2017) Meta-analysis of individual patient safety data from six randomized, placebo-controlled trials with the antiangiogenic VEGFR2-binding monoclonal antibody ramucirumab. Ann Oncol 28(12):2932–2942
Jung K, Heishi T, Incio J, Huang Y, Beech EY, Pinter M et al (2017) Targeting CXCR4-dependent immunosuppressive Ly6C(low) monocytes improves antiangiogenic therapy in colorectal cancer. Proc Natl Acad Sci U S A 114(39):10455–10460
Jenkins RW, Barbie DA, Flaherty KT (2018) Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 118(1):9–16
Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23:225–274
Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S (2013) Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 31:227–258
Nakamura K, Nakayama M, Kawano M, Amagai R, Ishii T, Harigae H et al (2013) Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc Natl Acad Sci U S A 110(23):9421–9426
López-Soto A, Huergo-Zapico L, Acebes-Huerta A, Villa-Alvarez M, Gonzalez S (2015) NKG2D signaling in cancer immunosurveillance. Int J Cancer 136(8):1741–1750
Nausch N, Cerwenka A (2008) NKG2D ligands in tumor immunity. Oncogene 27(45):5944–5958
Ghadially H, Brown L, Lloyd C, Lewis L, Lewis A, Dillon J et al (2017) MHC class I chain-related protein A and B (MICA and MICB) are predominantly expressed intracellularly in tumour and normal tissue. Br J Cancer 116(9):1208–1217
Schilling D, Kühnel A, Tetzlaff F, Konrad S, Multhoff G (2015) NZ28-induced inhibition of HSF1, SP1 and NF-κB triggers the loss of the natural killer cell-activating ligands MICA/B on human tumor cells. Cancer Immunol, Immunother : CII 64(5):599–608
de Ferrari Andrade L, Tay RE (2018) Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell–driven tumor immunity. Science 359(6383):1537–1542
Wang T, Sun F, Xie W, Tang M, He H, Jia X et al (2016) A bispecific protein rG7S-MICA recruits natural killer cells and enhances NKG2D-mediated immunosurveillance against hepatocellular carcinoma. Cancer Lett 372(2):166–178
Strong RK, McFarland BJ (2004) NKG2D and related immunoreceptors. Adv Protein Chem 68:281–312
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN et al (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545(7655):495–499
Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T et al (2018) Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res 78(16):4586–4598
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Xie W, Liu F, Wang Y, Ren X, Wang T, Chen Z et al (2016) VEGFR2 targeted antibody fused with MICA stimulates NKG2D mediated immunosurveillance and exhibits potent anti-tumor activity against breast cancer. Oncotarget 7(13):16445–16461
Xu Y, Zhang XR, Wang Y, Pan MZ, Wang M, Zhang J (2019) A VEGFR2-MICA bispecific antibody activates tumor-infiltrating lymphocytes and exhibits potent anti-tumor efficacy in mice. Cancer Immunol Immun 68(9):1429–1441
Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G et al (2006) A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124(6):1283–1298
Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294(1–2):15–22
Groh V, Wu J, Yee C, Spies T (2002) Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419(6908):734–738
Klöß S, Chambron N, Gardlowski T, Arseniev L, Koch J, Esser R et al (2015) Increased sMICA and TGFβ(1) levels in HNSCC patients impair NKG2D-dependent functionality of activated NK cells. Oncoimmunology 4(11):e1055993
Lee SJ, Lim KT (2008) Phytoglycoprotein inhibits interleukin-1β and interleukin-6 via p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated RAW 264.7 cells. Naunyn-Schmiedeberg’s Arch Pharmacol 377(1):45–54
Waldhauer I, Steinle A (2008) NK cells and cancer immunosurveillance. Oncogene 27(45):5932–5943
Pahl J, Cerwenka A (2017) Tricking the balance: NK cells in anti-cancer immunity. Immunobiology 222(1):11–20
Schmiedel D, Mandelboim O (2018) NKG2D Ligands-critical targets for cancer immune escape and therapy. Front Immunol 9:2040
Deguine J, Breart B, Lemaître F, Bousso P (2012) Cutting edge: tumor-targeting antibodies enhance NKG2D-mediated NK cell cytotoxicity by stabilizing NK cell-tumor cell interactions. J Immunol 189(12):5493–5497
Hu J, Bernatchez C, Zhang L, Xia X, Kleinerman ES, Hung MC et al (2017) Induction of NKG2D ligands on solid tumors requires tumor-specific CD8(+) T cells and histone acetyltransferases. Cancer Immunol Res 5(4):300–311
Alexander ET, Minton AR, Peters MC, van Ryn J, Gilmour SK (2016) Thrombin inhibition and cisplatin block tumor progression in ovarian cancer by alleviating the immunosuppressive microenvironment. Oncotarget 7(51):85291–85305
Sica A, Schioppa T, Mantovani A, Allavena P (2006) Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 42(6):717–727
Allen E, Jabouille A (2017) Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Trans Med 9(385):eaak9679
Tada Y, Togashi Y, Kotani D, Kuwata T, Sato E, Kawazoe A et al (2018) Targeting VEGFR2 with Ramucirumab strongly impacts effector/ activated regulatory T cells and CD8(+) T cells in the tumor microenvironment. J Immunother Cancer 6(1):106
Lu G, Nishio N, van den Berg NS, Martin BA, Fakurnejad S, van Keulen S et al (2020) Co-administered antibody improves penetration of antibody-dye conjugate into human cancers with implications for antibody-drug conjugates. Nat Commun 11(1):5667
Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR et al (2010) Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328(5981):1031–1035
Funding
This work was supported by the National Natural Science Foundation (NSFC81973223), and the National College Students Innovation and Entrepreneurship Training Program (202210316062Y, China).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. MP and FW designed and performed the experiments, carried out statistical analysis, and wrote the manuscript. LN guided anti-tumor efficacy in LLC model. SY, JQ, and HZ performed antibody-dependent cellular cytotoxicity assay, MTT assay, and helped with anti-tumoral activity in NSCLC model. JX and SS performed immunohistochemistry, PubMed searches and edited the manuscript. JZ proposed the study design, conducted the study supervision, and revised the manuscript. FS performed analysis and interpretation of data, and revised the manuscript. MW reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, M., Wang, F., Nan, L. et al. αVEGFR2-MICA fusion antibodies enhance immunotherapy effect and synergize with PD-1 blockade. Cancer Immunol Immunother 72, 969–984 (2023). https://doi.org/10.1007/s00262-022-03306-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03306-1