Abstract
Background
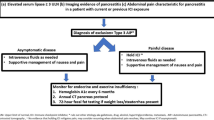
Drug-induced acute pancreatitis (AP) is uncommon and pancreatic involvement due to immune checkpoint inhibitors (ICI) in published reports relied on the National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Events (CTCAE). CTCAE definition of AP differs from the revised Atlanta classification diagnostic criteria. This study aims to classify the spectrum of pancreatic involvement in patients receiving ICI therapy into categories built on the revised Atlanta classification.
Methods
A retrospective cohort study of cancer patients receiving cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) inhibitors between 2011 and 2020. Pancreas-specific immune-related adverse events (irAEs) were categorized into AP and pancreatic injury.
Results
Forty-seven patients on ICI therapy met selection criteria. Twenty patients (43%) had AP, while 27 (57%) had pancreatic injury. Fifteen patients (75%) developed mild AP. Five patients progressed to pancreatic atrophy, and two patients (4%) developed exocrine pancreatic insufficiency. In both groups, most patients received nivolumab therapy (70% vs. 67%, p = 0.08) with no difference in mean number of nivolumab doses (9 vs. 10, p = 0.69). There was no correlation between the mean number of nivolumab or pembrolizumab doses and AP events (OR 0.94, p = 0.26, and OR 0.98, p = 0.86), but the duration of ICI therapy was significantly related to pancreatic atrophy (OR 1.01, p = 0.05; 95% CI 1.00–1.02).
Conclusion
Based on the novel classification, majority of pancreatic irAEs were classified as asymptomatic pancreatic injury but with some risk of pancreatic atrophy. This classification can help in assessing patterns of pancreatic involvement, pathogenesis, and treatment decisions.

Similar content being viewed by others
Abbreviations
- CTCAE:
-
Common terminology criteria for adverse events
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- EPI:
-
Exocrine pancreatic insufficiency
- ICI:
-
Immune checkpoint inhibitors
- irAEs:
-
Immune-related adverse events
- IRB:
-
Institutional review board
- NCI:
-
National cancer institute
- PD-1:
-
Programmed cell death protein 1
- UNL:
-
Upper normal limit
References
Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN (2018) Initial medical treatment of acute pancreatitis: American gastroenterological association institute technical review. Gastroenterology 154:1103–1139
Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN (2018) American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology 154:1096–1101
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG et al (2013) Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 62:102–111
Abu-Sbeih H, Tang T, Lu Y, Thirumurthi S, Altan M, Jazaeri AA, Dadu R et al (2019) Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J Immunother Cancer 7:31
Friedman CF, Clark V, Raikhel AV, Barz T, Shoushtari AN, Momtaz P, Callahan MK et al (2017) Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab + ipilimumab. J Natl Cancer Inst 109:djw260
Bajwa R, Cheema A, Khan T, Amirpour A, Paul A, Chaughtai S, Patel S et al (2019) Adverse effects of immune checkpoint inhibitors (programmed death-1 inhibitors and cytotoxic T-lymphocyte-associated protein-4 inhibitors): results of a retrospective study. J Clin Med Res 11:225–236
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Villadolid J, Amin A (2015) Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 4:560–575
Hsu C, Marshall JL, He AR (2020) Workup and management of immune-mediated hepatobiliary pancreatic toxicities that develop during immune checkpoint inhibitor treatment. Oncologist 25:105–111
Cramer P, Bresalier RS (2017) Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep 19:3
Callahan MK, Kluger H, Postow MA, Segal NH, Lesokhin A, Atkins MB, Kirkwood JM et al (2018) Nivolumab plus ipilimumab in patients with advanced melanoma: updated survival, response, and safety data in a phase I dose-escalation study. J Clin Oncol 36:391–398
Kohlmann J, Wagenknecht D, Simon JC, Ziemer M (2019) Immune-related pancreatitis associated with checkpoint blockade in melanoma. Melanoma Res 29:549–552
Michot JM, Ragou P, Carbonnel F, Champiat S, Voisin AL, Mateus C, Lambotte O et al (2018) Significance of immune-related lipase increase induced by antiprogrammed death-1 or death ligand-1 antibodies: a brief communication. J Immunother 41:84–85
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34
George J, Bajaj D, Sankaramangalam K, Yoo JW, Joshi NS, Gettinger S, Price C et al (2019) Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: a systematic review and meta-analysis. Pancreatology 19:587–594
Su Q, Zhang XC, Zhang CG, Hou YL, Yao YX, Cao BW (2018) Risk of immune-related pancreatitis in patients with solid tumors treated with immune checkpoint inhibitors: systematic assessment with meta-analysis. J Immunol Res 2018:1027323
Widmann G, Nguyen VA, Plaickner J, Jaschke W (2016) Imaging features of toxicities by immune checkpoint inhibitors in cancer therapy. Curr Radiol Rep 5:59
Thomas R, Sebastian B, George T, Majeed NF, Akinola T, Laferriere SL, Braschi-Amirfarzan M (2020) A review of the imaging manifestations of immune check point inhibitor toxicities. Clin Imaging 64:70–79
Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, Nishino M (2015) Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with Ipilimumab. Cancer Immunol Res 3:1185–1192
Prasanna T, McNeil CM, Nielsen T, Parkin D (2018) Isolated immune-related pancreatic exocrine insufficiency associated with pembrolizumab therapy. Immunotherapy 10:171–175
Ismail OZ, Bhayana V (2017) Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem 50:1275–1280
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I et al (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 36:1714–1768
Acknowledgements
The study had no funding support. Robert R. McWilliams received prior compensation from Zentalis Pharmaceuticals for advisory services. All other authors of this manuscript have no conflict of interest to disclose.
Author information
Authors and Affiliations
Contributions
MA and SSV contributed to conception and design; MA, HT, and SSV contributed to analysis and data interpretation; MA contributed to manuscript writing; MA, SC, HT, NT, RRM, and SSV contributed to critical revision of the article for important intellectual content; MA, SC, HT, NT, RRM, and SSV contributed to final approval of the article; MA and HT contributed to statistical expertise; MA, SC, and HT contributed to collection and assembly of data.
Corresponding author
Ethics declarations
Conflict of interest
Motaz Ashkar, Shruti Chandra, Hiroaki Takahash, Naoki Takahashi, Santhi Swaroop Vege—no conflict of interest to disclose and no project-related funding support. Robert R. McWilliams—Zentalis Pharmaceuticals advisor, no project-related funding.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashkar, M., Chandra, S., Vege, S.S. et al. Pancreatic involvement due to immune checkpoint inhibitors: a proposed classification. Cancer Immunol Immunother 72, 895–901 (2023). https://doi.org/10.1007/s00262-022-03295-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03295-1