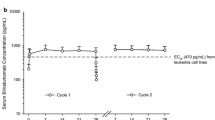
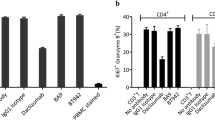
Abstract
High rates of relapse and poor prognosis confer an urgent need for novel therapeutic agents for B cell non-Hodgkin lymphomas (B-NHLs). Herein, we describe a human IgG-like anti-CD79b/CD3 bispecific antibody (IBI38D9-L) that selectively depletes antigen-positive malignant B cells as an alternative treatment option for relapsed or refractory NHL patients. The antitumor activity and mechanism of action of IBI38D9-L were investigated in vitro using B-NHL cell lines and human primary effector cells and in vivo using xenograft models reconstituted with human PBMCs (peripheral blood mononuclear cells). Pharmacokinetic (PK) properties and preclinical toxicology were evaluated in cynomolgus monkeys and HSC-NPG mice. IBI38D9-L exerted potent B cell killing as well as T cell activation and proliferation in a tumor cell-dependent manner in vitro and was active against B-NHL cell lines with various CD79b expression levels. Subcutaneous xenograft tumors in NOG mice engrafted with human PBMCs were eradicated by IBI38D9-L treatment. Moreover, IBI38D9-L-treated mice showed a strong infiltration of activated T cells. In HSC-NPG mice, IBI38D9-L resulted in potent B cell depletion in peripheral blood and induced only slight body weight loss and cytokine release syndrome without significant toxicological findings. In cynomolgus monkeys, IBI38D9-L was well tolerated with good pharmacokinetic profiles. Collectively, these preclinical efficacy and safety data provide strong scientific rationales for using anti-CD79b/CD3 bispecific antibody as a promising therapeutic agent for B cell malignancies.







Similar content being viewed by others
Abbreviations
- AAALAC:
-
Association for Assessment and Accreditation of Laboratory Animal Care International
- ADC:
-
Antibody–drug conjugates
- ALL:
-
Acute lymphoblastic leukemia
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- Bite:
-
Bispecific T cell engager
- B-NHLs:
-
B cell non-Hodgkin lymphomas
- BsAbs:
-
Bispecific antibodies
- CBA:
-
Cytometric Bead Array
- CREA:
-
Creatinine
- CRS:
-
Cytokine release syndrome
- DLBCL:
-
Diffuse large B cell lymphoma
- FDA:
-
Food and Drug Administration
- IFN:
-
Interferon
- IL:
-
Interleukin
- MFI:
-
Median fluorescence intensity
- OCT:
-
Optimal cutting temperature
- PBMCs:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate-buffered saline
- PE:
-
Phycoerythrin
- PK:
-
Pharmacokinetic
- PLT:
-
Platelet
- RBC:
-
Red blood cell
- sIg:
-
Surface Ig
- SPR:
-
Surface plasma resonance
- TGI:
-
Tumor growth inhibition
- TMDD:
-
Target-mediated drug disposition
- TNF:
-
Tumor necrosis factor
- WBC:
-
White blood cell
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71:7–33. https://doi.org/10.3322/caac.21654
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132. https://doi.org/10.3322/caac.21338
Ghielmini M (2005) Multimodality therapies and optimal schedule of antibodies: rituximab in lymphoma as an example. Hematol Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2005.1.321
Shankland KR, Armitage JO, Hancock BW (2012) Non-Hodgkin lymphoma. Lancet 380:848–857. https://doi.org/10.1016/S0140-6736(12)60605-9
Casulo C, Byrtek M, Dawson KL et al (2015) Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the national lymphocare study. J Clin Oncol 33:2516–2522. https://doi.org/10.1200/JCO.2014.59.7534
Gisselbrecht C, Glass B, Mounier N et al (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28:4184–4190. https://doi.org/10.1200/JCO.2010.28.1618
Matsuuchi L, Gold MR (2001) New views of BCR structure and organization. Curr Opin Immunol 13:270–277. https://doi.org/10.1016/s0952-7915(00)00215-6
Chu PG, Arber DA (2001) CD79: a review. Appl Immunohistochem Mol Morphol 9:97–106. https://doi.org/10.1097/00129039-200106000-00001
Zomas AP, Matutes E, Morilla R, Owusu-Ankomah K, Seon BK, Catovsky D (1996) Expression of the immunoglobulin-associated protein B29 in B cell disorders with the monoclonal antibody SN8 (CD79b). Leukemia 10:1966–1970
Cabezudo E, Carrara P, Morilla R, Matutes E (1999) Quantitative analysis of CD79b, CD5 and CD19 in mature B-cell lymphoproliferative disorders. Haematologica 84:413–418
Dornan D, Bennett F, Chen Y et al (2009) Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 114:2721–2729. https://doi.org/10.1182/blood-2009-02-205500
Ormhoj M, Scarfo I, Cabral ML et al (2019) Chimeric antigen receptor T cells targeting CD79b show efficacy in lymphoma with or without cotargeting CD19. Clin Cancer Res 25:7046–7057. https://doi.org/10.1158/1078-0432.CCR-19-1337
Sun LL, Ellerman D, Mathieu M et al (2015) Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med 7:287ra70. https://doi.org/10.1126/scitranslmed.aaa4802
Pillarisetti K, Edavettal S, Mendonca M et al (2020) A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood 135:1232–1243. https://doi.org/10.1182/blood.2019003342
Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA (2006) Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol 43:763–771. https://doi.org/10.1016/j.molimm.2005.03.007
Junttila TT, Li J, Johnston J et al (2014) Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res 74:5561–5571. https://doi.org/10.1158/0008-5472.CAN-13-3622-T
Zhang Y, Huo M, Zhou J, Xie S (2010) PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99:306–314. https://doi.org/10.1016/j.cmpb.2010.01.007
Hezareh M, Hessell AJ, Jensen RC, van de Winkel JG, Parren PW (2001) Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J Virol 75:12161–12168. https://doi.org/10.1128/JVI.75.24.12161-12168.2001
Polson AG, Yu SF, Elkins K et al (2007) Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood 110:616–623. https://doi.org/10.1182/blood-2007-01-066704
Caballero A, Katkere B, Wen XY, Drake L, Nashar TO, Drake JR (2006) Functional and structural requirements for the internalization of distinct BCR-ligand complexes. Eur J Immunol 36:3131–3145. https://doi.org/10.1002/eji.200636447
Engelberts PJ, Hiemstra IH, de Jong B et al (2020) DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine 52:102625. https://doi.org/10.1016/j.ebiom.2019.102625
Iwata Y, Sasaki M, Harada A et al (2019) Daily ascending dosing in cynomolgus monkeys to mitigate cytokine release syndrome induced by ERY22, surrogate for T-cell redirecting bispecific antibody ERY974 for cancer immunotherapy. Toxicol Appl Pharmacol 379:114657. https://doi.org/10.1016/j.taap.2019.114657
Neelapu SS, Locke FL, Bartlett NL et al (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377:2531–2544. https://doi.org/10.1056/NEJMoa1707447
He X, Klasener K, Iype JM, Becker M, Maity PC, Cavallari M, Nielsen PJ, Yang J, Reth M (2018) Continuous signaling of CD79b and CD19 is required for the fitness of Burkitt lymphoma B cells. EMBO J. https://doi.org/10.15252/embj.201797980
Shulzhenko N, Morgun A, Matzinger P (2009) Spontaneous mutation in the Cd79b gene leads to a block in B-lymphocyte development at the C’ (early pre-B) stage. Genes Immun 10:722–726. https://doi.org/10.1038/gene.2009.70
Kim JH, Kim WS, Ryu K, Kim SJ, Park C (2016) CD79B limits response of diffuse large B cell lymphoma to ibrutinib. Leuk Lymphoma 57:1413–1422. https://doi.org/10.3109/10428194.2015.1113276
Teachey DT, Rheingold SR, Maude SL et al (2013) Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 121:5154–5157. https://doi.org/10.1182/blood-2013-02-485623
Moore PA, Zhang W, Rainey GJ et al (2011) Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 117:4542–4551. https://doi.org/10.1182/blood-2010-09-306449
Zheng B, Fuji RN, Elkins K et al (2009) In vivo effects of targeting CD79b with antibodies and antibody-drug conjugates. Mol Cancer Ther 8:2937–2946. https://doi.org/10.1158/1535-7163.MCT-09-0369
Klinger M, Zugmaier G, Nagele V et al (2020) Adhesion of T cells to endothelial cells facilitates Blinatumomab-associated neurologic adverse events. Cancer Res 80:91–101. https://doi.org/10.1158/0008-5472.CAN-19-1131
Staflin K, Zuch de Zafra CL, Schutt LK et al (2020) Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody. JCI Insight. https://doi.org/10.1172/jci.insight.133757
Zuch de Zafra CL, Fajardo F, Zhong W et al (2019) Targeting multiple myeloma with AMG 424, a novel anti-CD38/CD3 bispecific T-cell-recruiting antibody optimized for cytotoxicity and cytokine release. Clin Cancer Res 25:3921–3933. https://doi.org/10.1158/1078-0432.CCR-18-2752
Mandikian D, Takahashi N, Lo AA et al (2018) Relative target affinities of T-cell-dependent bispecific antibodies determine biodistribution in a solid tumor mouse model. Mol Cancer Ther 17:776–785. https://doi.org/10.1158/1535-7163.MCT-17-0657
Wang Y, Ni H, Zhou S et al (2021) Tumor-selective blockade of CD47 signaling with a CD47/PD-L1 bispecific antibody for enhanced anti-tumor activity and limited toxicity. Cancer Immunol Immunother 70:365–376. https://doi.org/10.1007/s00262-020-02679-5
Labrijn AF, Janmaat ML, Reichert JM, Parren P (2019) Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov 18:585–608. https://doi.org/10.1038/s41573-019-0028-1
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10. https://doi.org/10.1016/j.immuni.2013.07.012
Hipp S, Voynov V, Drobits-Handl B, Giragossian C, Trapani F, Nixon AE, Scheer JM, Adam PJ (2020) A bispecific DLL3/CD3 IgG-like T-cell engaging antibody induces antitumor responses in small cell lung cancer. Clin Cancer Res 26:5258–5268. https://doi.org/10.1158/1078-0432.CCR-20-0926
Feucht J, Kayser S, Gorodezki D et al (2016) T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget 7:76902–76919. https://doi.org/10.18632/oncotarget.12357
Mimura K, Teh JL, Okayama H et al (2018) PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci 109:43–53. https://doi.org/10.1111/cas.13424
Acknowledgements
We thank all scientists in the Innovent CD79b/CD3 project team.
Funding
This study was supported by Innovent Biologics and grants from Suzhou Municipal Science and Technology Bureau (Grant Number SLJ202011), Jiangsu Commission of Health (Grant Number M2020043) and Special Technical Project of Diagnosis and Treatment of Key Clinical Diseases of Suzhou (Grant Number LCZX201813).
Author information
Authors and Affiliations
Contributions
JW, CL and KH designed the experiments, wrote and edited the manuscript. ZK performed the in vitro T cell activation, killing and cytokine release assay. JL performed the pre-toxicology study in HSC-NPG mice. YY, MW and YL designed and performed in vivo tumor growth and ex vivo TIL analysis experiments. FH, LL, XC and MX performed the hybridoma screening and antibody selection. NL, FF and SZ performed the antibody generation and engineering. ZW performed and interpreted all protein biophysical analyses. DK and XQ designed and oversaw GLP monkey studies. BC FG and WW conceived the study, assembled and interpreted all data and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Chen Li and Feng Guo have no potential conflicts of interest to disclose. All other authors are employees of Innovent Biologics. Innovent Biologics has filed patent applications of this work.
Consent for publication
All authors agree to publish this article.
Ethical approval
All mice experiments were performed in accordance with the regulations for care and use of laboratory animals at Innovent Biologics and were approved by the Institutional Animal Care and Use Committee. All monkey experiments were approved by IACUC and performed by WestChina-Frontier PharmaTech, according to the regulations of Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Li, C., He, K. et al. Characterization of anti-CD79b/CD3 bispecific antibody, a potential therapy for B cell malignancies. Cancer Immunol Immunother 72, 493–507 (2023). https://doi.org/10.1007/s00262-022-03267-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03267-5