Abstract
Background
TAS0313 is a multi-epitope long peptide vaccine targeting several cancer-associated antigens highly expressed in multiple cancer types, including glioblastoma (GBM). This cohort of a Phase 2 part evaluated the efficacy and safety of TAS0313 in patients with GBM.
Methods
TAS0313 (27 mg) was administered subcutaneously on Days 1, 8 and 15 of Cycles 1 and 2, and Day 1 of subsequent cycles in 21-day cycles. The primary endpoint was the objective response rate (ORR). The secondary endpoints were the disease control rate, progression-free survival (PFS) and 6- and 12-month progression-free survival rates (PFR) and safety. Immunological response was assessed as an exploratory endpoint.
Results
The best overall response was partial response in 1 patient, and the ORR (95% CI) was 11.1% (0.3–48.2%) in the per-protocol set (n = 9). A further 3 patients achieved stable disease, for a disease control rate (95% CI) of 44.4% (13.7–78.8%). Median (95% CI) PFS was 1.7 (1.3–NE) months and 6- and 12-month PFRs (95% CI) were 22.2% (3.4–51.3%) each. Common (≥ 20% incidence) treatment-related adverse events (AEs) were injection site reactions (n = 8, 80.0%), followed by pyrexia (n = 7, 70.0%), and malaise, injection site erythema and injection site pruritus (n = 2, 20.0% each). There were no grade 4 or 5 treatment-related AEs. No deaths occurred during the study. In some patients, TAS0313 treatment was confirmed to increase cytotoxic T lymphocyte and immunoglobulin G levels compared with baseline.
Conclusion
TAS0313, a multi-epitope long peptide vaccine, demonstrated promising efficacy and acceptable safety in patients with recurrent GBM.
Clinical trial registration
JapicCTI-183824 (Date of registration: Jan 11, 2018)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis for glioblastoma (GBM) remains dismal, with a 5-year survival rate of < 10% [1]. Despite multimodal treatment consisting of maximal safe surgical resection, followed by radiotherapy (RT) with temozolomide (TMZ), and adjuvant TMZ, [2] almost all patients experience recurrence [3]. Following recurrence, no standard of care exists and treatment options are limited [4, 5]. Bevacizumab has been shown to extend progression-free survival (PFS) in recurrent GBM (rGBM) but has failed to demonstrate a survival advantage [6, 7]. Similarly, clinical trials of several immunotherapy agents performed in the setting of rGBM have proven unsuccessful [8, 9]. Treatment needs therefore remain unmet, with even the latest immuno-oncology approaches not considered effective in rGBM.
Cancer peptide vaccines are a novel type of cancer immunotherapy that exert their antitumor effect via induction of cytotoxic T lymphocytes (CTLs) reactive to recognized cancer-associated antigens. To date, clinical studies of cancer peptide vaccines have typically used short peptides consisting of 8–10 amino acid residues, which have demonstrated partial efficacy in urothelial carcinoma and GBM [10,11,12,13]. However, despite promising preliminary results, the response rate across multiple clinical studies is only 2.9% [14] due to several factors [15,16,17].
TAS0313 is a novel multi-epitope long peptide vaccine cocktail comprising 3 long peptide chains (TAS0314, TAS0315 and TAS0316) that harbor 12 CTL epitope peptides. These peptides are derived from 8 cancer-associated antigens known to be highly expressed in multiple cancer types, including GBM [18].
A first-in-human phase 1/2 clinical study of TAS0313 has recently been conducted in patients with advanced solid tumors for whom no standard therapy is available. Results of the dose-finding portion of the study demonstrated promising preliminary safety and efficacy at both 9 mg and 27 mg doses [19]. We report the results from Cohort B of the study, which evaluated the efficacy and safety of TAS0313 monotherapy in patients with rGBM at the dose established in the dose-finding cohort [19].
Materials and methods
Study design and treatment
This phase 1/2 open-label, non-randomized, multicenter study evaluated the tolerability, safety and efficacy of TAS0313 in patients with solid tumors.
The study design consisted of 4 parts, including: a dose-finding cohort (Cohort A); an efficacy-finding cohort (Cohort B); and 2 additional cohorts, which evaluated the efficacy and safety of TAS0313 in combination with pembrolizumab in patients with urothelial carcinoma without (Cohort C1) and with (Cohort C2) prior exposure to immune checkpoint inhibitors. Data from the dose-finding cohort have been reported previously; [19] here, we present the results from the efficacy-finding cohort (Cohort B).
The primary objective of Cohort B was to evaluate the efficacy of TAS0313 monotherapy in patients with rGBM using the recommended dose (27 mg) determined in Cohort A of the phase 1/2 study. Safety was a secondary objective. Exploratory objectives included peptide-specific CTL, peptide-specific immunoglobulin G (IgG), tumor-infiltrating lymphocyte (TIL) counts, blood cytokine concentrations in plasma samples and mRNA expression levels of immunological factors and target cancer-associated antigens from archival tumor tissue samples obtained by surgical resection.
The study drug was prepared by dissolving 27 mg lyophilized TAS0313 in water, which was then mixed with an adjuvant, Montanide™ ISA 51 VG, at a ratio of 1:1 for emulsification. The study drug emulsion was administered subcutaneously near the lymph node (upper back, axillary, inguinal or abdominal) on Days 1, 8 and 15 of Cycles 1 and 2, and on Day 1 of subsequent cycles in 21-day cycles.
The study was conducted in accordance with ethical principles of the Declaration of Helsinki, the International Council for Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice and Institutional Review Board regulations. All patients provided written informed consent to participate in the study.
Patients
Patients aged between 20 and 70 years with histologically confirmed Grade IV rGBM (including gliosarcoma, giant cell glioblastoma and epithelioid glioblastoma) as per World Health Organization classification criteria [20] who had either the HLA-A*02:01, HLA-A*02:06, HLA-A*02:07, HLA-A*11:01, HLA-A*24:02, HLA-A*31:01 or HLA-A*33:03 allele type were eligible.
Additional eligibility criteria included: confirmed first or second recurrence, or disease progression (PD) following standard therapy with RT and TMZ; ≥ 1 measurable lesion based on Response Assessment in Neuro-Oncology (RANO) criteria [21] by magnetic resonance imaging (MRI); a Karnofsky Performance Scale (KPS) score of ≥ 70 at enrollment; adequate hematological (absolute neutrophil count of ≥ 1500/mm3; hemoglobin value of ≥ 8.0 g/dL; platelet count of ≥ 75,000/mm3), renal [serum creatinine ≤ 1.5× upper limit of normal (ULN) or creatinine clearance of ≥ 50 mL/min] and liver (aspartate aminotransferase and alanine aminotransferase ≤ 3× ULN, total bilirubin ≤ 1.5× ULN) function; life expectancy of ≥ 3 months.
Outcome measures
Efficacy
The primary efficacy endpoint was the objective response rate [ORR; defined as the proportion of patients achieving complete response (CR) or partial response (PR)], as per RANO [21] and iRANO [22] criteria.
Secondary efficacy endpoints included the disease control rate (DCR), duration of response, PFS and PFS rate (PFR) as per RANO [21] and iRANO [22] criteria. Disease control rate was defined as the percentage of patients with a best overall response of CR, PR or SD. Duration of response was defined as the period from the date of CR or PR until the date of confirmation of PD or death due to any cause, whichever occurred first. PFS was defined as the period from the date of enrollment until the date of PD or death due to any cause, whichever occurred first. Patients without any PD event at the time of analysis or receiving subsequent treatment were censored at the date of last evaluation that confirmed absence of PD. PFR was defined as the proportion of patients without PD at 6 or 12 months after the date of enrollment (6- and 12-month PFR, respectively). Tumors were assessed using MRI scans at baseline, then every 6 weeks from Day 1 of Cycle 1.
Safety
Safety assessments included the incidence of AEs, including serious AEs, treatment-related AEs and laboratory variables. AEs were defined as any unfavorable or unintended sign, symptom or illness occurring in a patient enrolled in the study, irrespective of its relationship to the study drug. Treatment-related AEs were defined as any AE for which a causal relationship to the study drug could not be denied. AEs were evaluated and categorized by system organ class (SOC) and preferred term (PT) using MedDRA version 23.1, with severity graded according to the Common Terminology Criteria for Adverse Events version 4.03.
As part of the safety assessment, data were also collected on vital signs, body weight, laboratory variables and 12-lead electrocardiogram findings.
Biomarker analysis
Peptide-specific CTL counts and peptide-specific IgG antibody concentrations were measured from blood samples collected before TAS0313 treatment (≤ 1 week before enrollment) and at Day 22 of Cycle 2 and Cycle 3; full experimental details are provided in Supplementary Table 1. CTL and IgG induction were analyzed to evaluate the pharmacodynamics of TAS0313. CTL induction was defined as patients who had ≥ 180 spots/100,000 cells based on the difference in mean + 3× standard deviation between the negative control samples of the overall patient population.
Tumor-infiltrating CD8+ lymphocyte count was measured from either formalin-fixed, paraffin-embedded tumor tissue samples before TAS0313 treatment (≤ 28 days before enrollment) or archival tumor tissue samples (mandatory), and at Day 22 of Cycle 2 (optional). Tumor-infiltrating CD8+ T lymphocytes in formalin-fixed, paraffin-embedded tissues were evaluated by immunohistochemical staining of slides with anti-CD8 (SP57) rabbit monoclonal primary Ab (Roche Diagnostics) and Ventana iVIEW DAB Universal Kit (Roche Diagnostics) according to the manufacturer’s instructions. Two fields in hotspots with CD8+ T cell infiltration at the tumor site were selected and CD8+ cells were counted.
Cytokine concentrations were measured from plasma samples collected before TAS0313 treatment (≤ 1 week before enrollment).
mRNA expression levels of immunological factors and target cancer-associated antigens were measured from archival tumor tissue samples obtained by surgical resection by Riken Genesis using a modified nCounter PanCancer IO 360 Gene Expression Panel (nanoString) as per the manufacturer’s instructions. Measurement of TAS0313 target cancer antigens was carried out in this modified panel by adding sets of probes. Human HLA-A*03:01 cDNA and Human HLA-A*24:02:01 cDNA were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan [23].
Statistical analysis
The primary efficacy endpoint was evaluated using the per-protocol set (PPS), which included all enrolled patients who received TAS0313 on Days 1, 8 and 15 in Cycles 1 and 2 and had ≥ 1 post-treatment response evaluation available. Secondary analysis of the primary efficacy endpoint was evaluated using the full analysis set (FAS), which comprised all enrolled patients who received ≥ 1 dose of study drug. Secondary efficacy endpoints were evaluated using the FAS and PPS. Efficacy analyses were performed using a data cutoff date of 10 Sept 2020. Safety analyses were performed using the safety analysis set, which comprised all patients who received ≥ 1 dose of study drug. The pharmacodynamic-evaluable population included all patients who received ≥ 1 dose of study drug and had relevant data (e.g., CTL, IgG, tumor-infiltrating CD8+ TIL) available. The pharmacogenomics-evaluable population included all patients who received ≥ 1 dose of study drug and had relevant data (e.g., cytokine concentration, mRNA expression of immunological factors and target cancer-associated antigen) available.
Baseline demographics were summarized using descriptive statistics, with the mean, standard deviation and median (min, max) calculated for continuous variables and the frequency number and proportion calculated for categorical variables. Time-to-event analyses (PFS and 6- and 12-month PFR) were summarized using the Kaplan–Meier method with 95% CI. A post hoc analysis was also conducted, in which receiver operating curves (ROC) were generated to evaluate TIL count and IgG concentration cutoffs predictive of best response, with PR and SD represented as “positive” and PD represented as “negative” responses.
The frequency of adverse events was summarized descriptively overall and for each individual event (by SOC and PT).
All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient disposition
A total of 10 patients were enrolled into Cohort B of the study between March 12, 2019, and March 30, 2020. All (n = 10) patients were included in the FAS, safety analysis set, the pharmacodynamic- and pharmacogenomics-evaluable populations, respectively. Nine patients were included in the PPS.
Among the safety analysis set, 8 (80.0%) patients discontinued treatment with TAS0313, most frequently due to PD (n = 7, 87.5%). One patient discontinued treatment due to an AE (anaphylactoid reaction, 12.5%).
Patient characteristics and treatment
Baseline demographic and clinical characteristics of patients in the FAS are presented in Table 1. No significant differences in baseline demographics and clinical characteristics (including sex, age, KPS, prior surgery/treatment and HLA allele) were observed between patients in the FAS and PPS (data not shown).
TAS0313 treatment
The median (min, max) treatment duration was 70.0 (29, 393) days, the median (min, max) number of treatment cycles was 3.0 (2.0, 18.0), and the median (min, max) total administered dose was 189.0 (108, 594) mg.
Efficacy
The ORR (95% CI) in the PPS (the primary endpoint) was 11.1% (0.3–48.2%). The best overall response was PR in 1 (11.1%) patient, SD in 3 (33.3%) patients and PD in 5 (55.6%) patients according to RANO. Disease control was achieved in 4 patients, and the DCR (95% CI) was 44.4% (13.7–78.8%).
The ORR (95% CI) in the FAS (secondary analysis of the primary efficacy endpoint) was 10.0% (0.3–44.5%). The best overall response was PR in 1 (10.0%) patient, SD in 4 (40.0%) patients and PD in 5 (50.0%) patients according to RANO. Disease control was achieved in 5 patients, and the DCR (95% CI) was 50.0% (18.7–81.3%).
No difference in response rates were observed in the PPS and FAS when analyzed according to iRANO criteria.
The patient (patient number: B27-007) who achieved PR was aged 49 years, had HLA-A*24:02 allele, a KPS score of 80, MGMT promoter methylation, and was IDH wild type. The patient had failed multiple prior therapies following partial resection, including a combination of TMZ and nivolumab with/without RT, and bevacizumab and eribulin monotherapy. Following TAS0313 treatment, the patient achieved PR by Week 18, which persisted to beyond Week 36. This coincided with a reduction in tumor volume by Week 6, which reached 69.1% by Week 36 (Fig. 1).
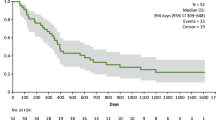
The Kaplan–Meier estimate of PFS in the PPS is presented in Fig. 2. Median (95% CI) PFS was 1.7 (1.3–NE) months and the 6- and 12-month PFR (95% CI) was 22.2% (3.4–51.3%) at each time point. Notably, 3 patients achieved PFS of ≥ 3.5 months and 2 (22.2%) patients continued TAS0313 treatment for over 8 months (Fig. 3). The median PFS (95% CI) in the FAS was 2.3 (1.3–NE) months, and the 6- and 12-month PFR (95% CI) was 25.0% (4.1–54.8%) at each time point.
Safety
The incidence of AEs and treatment-related AEs occurring during TAS0313 treatment are presented in Table 2.
A total of 10 (100.0%) patients experienced AEs during the study, 2 (20.0%) of which were grade ≥ 3 in severity. The most common treatment-related AE by PT was injection site reactions, occurring in 8 (80.0%) patients, followed by pyrexia (n = 7, 70.0%), and malaise, injection site erythema and injection site pruritus (n = 2, 20.0% each). Dermatological injection site reactions (Supplementary Table 2) occurred in 9 (90.0%) of patients and included injection site reaction (n = 8, 80.0%), injection site erythema (n = 2, 20.0%), injection site pruritus (n = 2, 20.0%), injection site pain (n = 1, 10.0%), injection site abscess (n = 1, 10.0%), injection site swelling (n = 1, 10.0%) and injection site injury (n = 1, 10.0%).
All treatment-related AEs were grade 1 or grade 2 in severity, with the exception of 1 (10.0%) patient, who experienced a grade ≥ 3 anaphylactoid reaction. This event resolved following appropriate treatment with electrolyte solution and antihistamines and TAS0313 discontinuation. No deaths occurred during the study. No other patients discontinued treatment due to AEs, and no patients experienced dose interruptions due to AEs.
Biomarker analysis
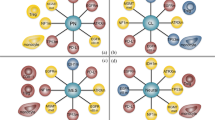
We analyzed the induction of CTL and IgG to evaluate the pharmacodynamics of TAS0313. CTL induction was confirmed in 6 (60.0%) patients (Table 3). Variation in CTL counts before and after TAS0313 administration by HLA type is presented in Fig. 4.
Variation in cytotoxic T lymphocyte counts following TAS0313 administration by HLA Typea (pharmacogenomic-evaluable population). aDays from date of first treatment = (measurement date) − (date of first administration) + 1. When change from baseline was less than zero, it was treated as zero. Patients evaluable for pharmacodynamics analysis were all treated patients who had available data on CTL, IgG or tumor-infiltrating CD8+ T lymphocytes. CTL, cytotoxic T lymphocyte; HLA, human leukocyte antigen; IgG, immunoglobulin G
CTL counts increased significantly compared with baseline in the patient with the HLA-A*24 allele type, reaching a peak of ~ 1200 spots/100,000 cells after Day 49, before declining to Day 80, although counts remained above 200 spots/100,000 cells at all time points. Although less pronounced, patients with the HLA-A*2, HLA-A*A11, HLA-A*31, HLA-A*33 alleles also experienced gradual but persistent increases in CTL counts following TAS0313 administration.
IgG induction, defined as patients with an elevation in IgG of ≥ 30% to at least 1 epitope in TAS0313 following treatment compared with baseline, was confirmed in all 10 (100.0%) patients. Induction of IgG to all peptides was observed regardless of antigen type (Supplementary Table 3).
A correlation between immunological response and efficacy was observed in 2 patients (Table 3), as evidenced by an IgG level ≥ 30% and CTL count ≥ 180 spots/100,000 cells, which correlated with prolonged PFS (ongoing at 12.7 months and 8.3 months, respectively). However, both IgG and CTL levels were not significantly higher in the 2 patients with prolonged PFS compared with the other 4 patients (Supplementary Table 3, Fig. 4). No trend was observed between injection site reaction and pyrexia severity and degree of immunological response.
To further examine the patient populations in which efficacy of TAS0313 was observed, the correlation between baseline biomarkers and efficacy was analyzed.
For the post hoc ROC analysis, the ideal cutoff value of TIL for best overall response was calculated as 87. Patients with a TIL count ≥ 87 (n = 4) had prolonged PFS and superior responses [median PFS (95% CI), NR (4.0–NR); PR, n = 1 (25.0%); SD, n = 3 (75.0%)] compared with patients with a TIL count < 87 (n = 5) [median PFS (95% CI), 1.4 (1.3–1.7) months; PD, n = 5 (100.0%)] (Fig. 5). Each score of immune-related cells in pretreatment archival tumor tissue samples was measured by nCounter. In addition to CD8+ T cells, the population difference of many immune-related cells was observed. The score of macrophages was significantly high, the score of myeloid cells including MDSC and Treg also tended to be high in cases of PR and SD (data not shown).
Progression-free survival in patients with recurrent glioblastoma according to tumor-infiltrating lymphocyte count (pharmacodynamic-evaluable population a). aTen patients had pharmacodynamic data available and were included in this analysis. CR, complete response; PD, disease progression; PFS, progression-free survival; PR, partial response; SD, stable disease
The relationship between peptide-specific IgG concentrations of the pre-treated sample and tumor volume change (%) from baseline were also evaluated. Twelve baseline IgG cutoff values were established by applying ROC curve analysis, and all IgG concentrations were defined as having met or not met the cutoff criteria (cutoff criteria, TA1: ≥ 1748.75 pg/mL, TA2: ≥ 9710.43 pg/mL, TA4: ≤ 93,507.37 pg/mL, TA5: ≥ 6404.04 pg/mL, TA6: ≥ 3334.06 pg/mL, TA7: ≥ 33,638.80 pg/mL, TA8: ≥ 25,796.77 pg/mL, TA9: ≥ 53,945.56 pg/mL, TA10: ≥ 12,772.04 pg/mL, TA13: ≥ 4345.17 pg/mL, TA15: ≥ 2901.76 pg/mL, TA18: ≥ 1643.41 pg/mL). Of the cutoff criteria, TA5, TA6, TA8, TA9 and TA10 showed superior sensitivity and specificity (≥ 70%) (Supplementary Table 4). Interestingly, tumor shrinkage in the patient with baseline preexisting IgG to ≥ 6 peptides that met the cutoff criteria was significantly better than in the other patients, suggesting that peptide-specific IgG positivity prior to TAS0313 treatment potentially contributed to antitumor activity (Fig. 6).
Cytokine concentrations and mRNA expression of immunological factors and target cancer-associated antigens at baseline in patients receiving TAS0313 are presented in Supplementary Tables 5–8. The mRNA expression of almost all target cancer‐related antigens and HLA-A were confirmed in all enrolled patients (Supplementary Table 5). No correlation was found between mRNA expression of immunological factors within the tumor and PFS or blood cytokine concentrations and PFS, respectively (Table 3 and Supplementary Tables 5–8).
Discussion
In this efficacy-finding cohort (Cohort B) of a phase 1/2 study, the promising efficacy of TAS0313 was shown in adult patients with rGBM. The ORR (primary endpoint) was 11.1%, and marked tumor shrinkage was confirmed in 1 patient who achieved PR. This patient achieved PR by Week 18 of TAS0313 treatment, which was maintained to beyond Week 36. MRI examination revealed a corresponding reduction in tumor volume by Week 6, with a clinically significant reduction of 69.1% by Week 36. Further, TAS0313 resulted in stabilization of disease in 3 (33.3%) patients in the PPS, for a DCR of 44.4%. The median PFS was 1.7 months, and the 6- and 12-month PFR was 22.2% at each time point. Three patients achieved PFS ≥ 3.5 months. Taken together, these findings suggest that TAS0313 has promising activity in inducing tumor shrinkage and suppressing fast-growing GBM tumor growth for an extended period of time.
The recommended dose evaluated in this cohort was 27 mg based on an absence of DLTs observed at this dose during the dose-finding cohort of this study [19]. Importantly, no major safety concerns were identified at this dose, and the safety profile was consistent with the findings reported in the dose-finding cohort of the study [19]. AEs occurred in all patients (n = 10, 100.0%); injection site reactions were the most common AE, occurring in 80.0% of patients, all of which were grade ≤ 2 in severity and manageable without TAS0313 discontinuation. Other common treatment-related AEs (occurring with an incidence of ≥ 20%) included pyrexia (n = 7, 70.0%), and malaise, injection site erythema and injection site pruritus (n = 2, 20.0% each). Dermatological injection site reactions, comprising injection site reaction, injection site erythema, injection site pruritus, injection site pain, injection site abscess, injection site swelling and injection site injury, occurred in 9 (90.0%) patients. One patient (10.0%) experienced a grade ≥ 3 treatment-related AE (anaphylactoid reaction), which resolved immediately without any symptoms of shock and necessitated TAS0313 discontinuation. No deaths occurred during the study.
Treatment options following rGBM remain challenging. Bevacizumab was approved by the Food and Drug Administration in 2017 for the treatment of rGBM on the basis of encouraging phase 1/2 data, [6] but subsequent studies failed to demonstrate prolongation of OS, and unmet needs remain [7]. The promising preliminary efficacy and favorable safety profile observed in the current study suggest that TAS0313 may present a more attractive alternative therapy prior to bevacizumab. Notably, the efficacy of this vaccine was comparable with, or superior to, other peptide vaccines for GBM [24, 25]. However, further investigation aimed at identifying those patients most likely to derive greatest benefit from TAS0313 is warranted.
Consistent with the findings from the dose-finding portion of the study, [19] treatment with TAS0313 27 mg was associated with induction of CTL and IgGs compared with pretreatment, thus confirming the immune activation effects of TAS0313. Although the increase in CTL counts was most pronounced in the patient with the HLA-A*24 allele type (peak of ~ 1200 spots/100,000 cells after Day 49), patients with other alleles also experienced gradual but persistent increases in CTL counts following TAS0313 administration. These findings suggest that TAS0313 may be an effective treatment for multiple HLA phenotypes.
In patients with positive CTL and IgG in our study, long-term PFS was observed in 1 patient with PR (B27-007) and 1 patient with SD (B27-004) (Table 3). This is in contrast to the dose-finding portion of the study, [19] which found no clear relationship between immune and clinical responses. Changes in TIL counts after treatment could not be evaluated because biopsy of the tumor specimen could not be performed.
A TIL count of ≥ 87 at baseline appeared to be predictive of prolonged PFS and higher DCR in the post hoc analysis (Fig. 5). A correlation between TIL and PFS was observed in patients with rGBM in our study, which is consistent with the results reported in studies with other immunotherapy agents [26, 27]. Specifically, patients in the group with a high TIL count before TAS0313 vaccination experiencing significantly prolonged PFS versus those with a low TIL count. In contrast, TIL was not associated with prolonged survival in patients not receiving immunotherapy in similar studies in GBM [27]. Tumors with low TIL counts are recognized as having an immunosuppressive microenvironment that excludes immune cells to around the tumor and prevents them from infiltrating the tumor microenvironment [28]. Although the exact reason why TIL count correlates with prognosis is unclear, tumors with preexisting lymphocytic infiltration can be expected to have a favorable microenvironment for the immune system. Therefore, in tumors with high TIL counts, CTL induced by TAS0313 administration may efficiently migrate into the tumor and exert effector activity. The conditions for CTL, IgG, TIL and antigen expression were optimal and consistent with the known mechanism of action of TAS0313 in the 2 patients with prolonged PFS. No clear relationship was found between cytokine profile or mRNA expressions of immunological factors and prognosis.
The appropriate peptides for each patient in the ITK-1 study were chosen based on the peptide-specific IgG titer at baseline, expecting activation of secondary immune responses against tumor antigen [25]. On the other hand, it has been reported that even if IgG to the peptide cannot be detected before treatment, if IgG is induced after treatment, antitumor effects can be obtained [29]. The relationship between IgG antibody titers before treatment and the clinical effectiveness of peptide vaccines has been variously reported and remains unclear, but findings from the current study suggest that peptide-specific IgG concentrations at baseline were a predictive marker of tumor shrinkage. Since TAS0313 included several ITK-1 peptides, stratification of patients with high peptide-specific IgG concentration prior to treatment is likely to lead to better outcomes in future clinical trials. In addition, SART2 (TA5), SART3 (TA6, TA9) and Lck (TA8, TA10) demonstrated excellent sensitivity and specificity among the cutoff criteria for each IgG set in this study and may be independent predictors of efficacy. Conversely, as shown in Fig. 6, the more IgG types that met the cutoff criteria, the better the antitumor effect. However, further study is warranted.
This study also had some limitations that are inherent to phase 1/2 studies and must be considered. The sample size was small (n = 10) and the study was conducted in Japanese patients. Therefore, extrapolation of these results to other patient populations should be made with caution. Further, an independent control arm was not included as part of this study due to the relative rarity of GBM, making comparisons difficult. A comparative study is therefore warranted in future and is necessary to confirm that TIL and IgG are not prognostic predictors but rather markers of TAS0313 efficacy.
Nevertheless, the results of the efficacy-finding portion of this phase 1/2 study demonstrated the promising efficacy and manageable safety of TAS0313, a multi-epitope long peptide vaccine targeting several cancer-associated antigens, in adult patients with rGBM, a patient population with extremely poor prognosis and limited treatment options. The promising efficacy and manageable safety findings observed in this study support the further study of TAS0313 in patients with rGBM.
Data availability
Data will not be shared according to the Sponsor policy on data sharing.
Change history
08 May 2022
Corrections have been made in Table 3 and Figure 4 caption
Abbreviations
- AE:
-
Adverse event
- CR:
-
Complete response
- CTCAE:
-
Common terminology criteria for adverse events
- CTL:
-
Cytotoxic T lymphocyte
- DCR:
-
Disease control rate
- DLT:
-
Dose-limiting toxicity
- DOR:
-
Duration of response
- EGFR-vIII:
-
Epidermal growth factor receptor variant III
- FAS:
-
Full analysis set
- GBM:
-
Glioblastoma
- HLA:
-
Human leukocyte antigen
- IDH:
-
Isocitrate dehydrogenase
- IgG:
-
Immunoglobulin G
- iRANO:
-
Immunotherapy response assessment in neuro-oncology
- KPS:
-
Karnofsky performance scale
- MGMT:
-
O6-Methylguanine-DNA methyltransferase
- MRI:
-
Magnetic resonance imaging
- mRNA:
-
Messenger ribonucleic acid
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD:
-
Disease progression
- PFR:
-
Progression-free survival rate
- PFS:
-
Progression-free survival
- PPS:
-
Per-protocol set
- PR:
-
Partial response
- PT:
-
Preferred term
- RANO:
-
Response assessment in neuro-oncology
- rGBM:
-
Recurrent GBM
- ROC:
-
Receiver operating curve
- RT:
-
Radiotherapy
- SD:
-
Stable disease
- SOC:
-
System organ class
- TIL:
-
Tumor-infiltrating lymphocyte
- TL:
-
Target lesion
- TMZ:
-
Temozolomide
- ULN:
-
Upper limit of normal
- WHO:
-
World Health Organization
References
Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM (2020) Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep 10(1):11622. https://doi.org/10.1038/s41598-020-68011-4
Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G (2014) High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:iii93–iii101. https://doi.org/10.1093/annonc/mdu050
Bette S, Barz M, Huber T et al (2018) Retrospective analysis of radiological recurrence patterns in glioblastoma, their prognostic value and association to postoperative infarct volume. Sci Rep 8(1):4561. https://doi.org/10.1038/s41598-018-22697-9
Tan AC, Ashley DM, López GY et al (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70(4):299–312. https://doi.org/10.3322/caac.21613
Birzu C, French P, Caccese M et al (2020) Recurrent glioblastoma: from molecular landscape to new treatment perspectives. Cancers. https://doi.org/10.3390/cancers13010047
Wick W, Gorlia T, Bendszus M et al (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377(20):1954–1963. https://doi.org/10.1056/NEJMoa1707358
Gramatzki D, Roth P, Rushing EJ et al (2018) Bevacizumab may improve quality of life, but not overall survival in glioblastoma: an epidemiological study. Ann Oncol 29(6):1431–1436. https://doi.org/10.1093/annonc/mdy106
Weenink B, French PJ, Sillevis Smitt PAE, Debets R, Geurts M (2020) Immunotherapy in glioblastoma: current shortcomings and future perspectives. Cancers 12(3):751. https://doi.org/10.3390/cancers12030751
Reardon DA, Brandes AA, Omuro A et al (2020) Effect of nivolumab versus bevacizumab in patients with recurrent glioblastoma: the checkmate 143 phase 3 randomized clinical trial. JAMA Oncol 6(7):1003–1010. https://doi.org/10.1001/jamaoncol.2020.1024
Noguchi M, Matsumoto K, Uemura H et al (2016) An open-label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin Cancer Res 22(1):54–60. https://doi.org/10.1158/1078-0432.Ccr-15-1265
Terasaki M, Shibui S, Narita Y et al (2011) Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen–A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol 29(3):337–344. https://doi.org/10.1200/jco.2010.29.7499
Hashimoto N, Tsuboi A, Kagawa N et al (2015) Wilms tumor 1 peptide vaccination combined with temozolomide against newly diagnosed glioblastoma: safety and impact on immunological response. Cancer Immunol Immunother 64(6):707–716. https://doi.org/10.1007/s00262-015-1674-8
Izumoto S, Tsuboi A, Oka Y et al (2008) Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg 108(5):963–971. https://doi.org/10.3171/jns/2008/108/5/0963
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10(9):909–915. https://doi.org/10.1038/nm1100
Melief CJM, van der Burg SH (2008) Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 8(5):351–360. https://doi.org/10.1038/nrc2373
Hailemichael Y, Dai Z, Jaffarzad N et al (2013) Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med 19(4):465–472. https://doi.org/10.1038/nm.3105
Middleton D, Williams F, Meenagh A et al (2000) Analysis of the distribution of HLA-A alleles in populations from five continents. Hum Immunol 61(10):1048–1052. https://doi.org/10.1016/S0198-8859(00)00178-6
Tanaka Y, Wada H, Goto R et al (2020) TAS0314, a novel multi-epitope long peptide vaccine, showed synergistic antitumor immunity with PD-1/PD-L1 blockade in HLA-A*2402 mice. Sci Rep 10(1):17284. https://doi.org/10.1038/s41598-020-74187-6
Kondo S, Shimizu T, Koyama T et al (2021) First-in-human study of the cancer peptide vaccine TAS0313 in patients with advanced solid tumors. Cancer Sci 112(4):1514–1523. https://doi.org/10.1111/cas.14765
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972. https://doi.org/10.1200/jco.2009.26.3541
Okada H, Weller M, Huang R et al (2015) Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16(15):e534–e542. https://doi.org/10.1016/s1470-2045(15)00088-1
Akatsuka Y, Goldberg TA, Kondo E et al (2002) Efficient cloning and expression of HLA class I cDNA in human B-lymphoblastoid cell lines. Tissue Antigens 59(6):502–511. https://doi.org/10.1034/j.1399-0039.2002.590607.x
Fujimaki T, Itoh K, Terasaki M et al (2017) Trials of a personalized peptide vaccine (ITK-1) for patients with recurrent or progressive glioblastoma (GBM). Neuro Oncol 19(Suppl 3):iii52–iii52. https://doi.org/10.1093/neuonc/nox036.187
Narita Y, Arakawa Y, Yamasaki F et al (2018) A randomized, double-blind, phase III trial of personalized peptide vaccination for recurrent glioblastoma. Neuro Oncol 21(3):348–359. https://doi.org/10.1093/neuonc/noy200
Wheeler CJ, Black KL, Liu G et al (2008) Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 68(14):5955–5964. https://doi.org/10.1158/0008-5472.Can-07-5973
Hsu MS, Sedighim S, Wang T et al (2016) TCR sequencing can identify and track glioma-infiltrating T cells after DC vaccination. Cancer Immunol Res 4(5):412–418. https://doi.org/10.1158/2326-6066.Cir-15-0240
Wang M, Wang S, Desai J, Trapani JA, Neeson PJ (2020) Therapeutic strategies to remodel immunologically cold tumors. Clin Transl Immunol 9(12):e1226. https://doi.org/10.1002/cti2.1226
Oji Y, Hashimoto N, Tsuboi A et al (2016) Association of WT1 IgG antibody against WT1 peptide with prolonged survival in glioblastoma multiforme patients vaccinated with WT1 peptide. Int J Cancer 139(6):1391–1401. https://doi.org/10.1002/ijc.30182
Acknowledgements
The authors thank all clinicians for their involvement and contribution to the study. Medical writing assistance was provided by Jordana Campbell, BSc, CMPP of in Science Communications, Springer Healthcare. This medical writing assistance was funded by Taiho Pharmaceutical Co., Ltd.
Funding
This study was funded by Taiho Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Contributions
YN was involved in the study design. All authors participated in the drafting, critical revision and approval of the final version of the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Conflict of interest
YN has received grant support from Taiho Pharmaceutical Co., Ltd to their institution for manuscript preparation, has received grants from Taiho Pharmaceutical Co., Ono Pharmaceutical, Taiho Pharmaceutical Co., Eisai, Daiichi Sankyo, Stella-Pharma, Ohara, Denba and AbbVie to their institution in the past 3 years, has received consulting fees from AbbVie in the past 3 years and has received payments or honoraria from Chugai, Ono Pharmaceutical, Daiichi Sankyo, Eisai and Novocure in the past 3 years. YO has no conflicts of interest to disclose. YA has received grant support from Taiho Pharmaceutical Co., Ltd to their institution for manuscript preparation, has received grants from Siemens, Philips, Sanofi, Ono Pharmaceutical, Sanofi, Nihon Medi Physics, Brainlab, Carl Zeiss, Tanabe Mitsubishi, Chugai, Eisai, Merck, Meiji Seika, Daiichi Sankyo, CSL Behring, Takeda, Pfizer, Stryker and Astellas Pharma to their institution in the past 3 years and has received payments or honoraria from Nippon Kayaku, Novocure, UCB Japan, Ono Pharmaceutical, Brainlab, Chugai, Merck, Eisai, Meiji Seika, Daiichi Sankyo, CSL Behring, Integra Japan, Carl Zeiss, Otsuka and AbbVie in the past 3 years.
Consent to participate
All patients provided written informed consent to participate in the study.
Ethics approval
The clinical study protocol, investigator’s brochure, a sample informed consent form and other study-related documents were reviewed and approved by the local of all study sites. Each investigator conducted the study according to applicable local or regional regulatory requirements and in accordance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice and Institutional Review Board regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Narita, Y., Okita, Y. & Arakawa, Y. Evaluation of the efficacy and safety of TAS0313 in adults with recurrent glioblastoma. Cancer Immunol Immunother 71, 2703–2715 (2022). https://doi.org/10.1007/s00262-022-03184-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03184-7