Abstract
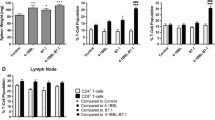
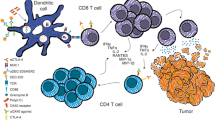
Dendritic cells are potently activated by the synergistic action of CD40 stimulation in conjunction with signaling through toll like receptors, subsequently priming T cells. Cancer vaccines targeting the activation of dendritic cells in this manner show promise in murine models and are being developed for human patients. While the efficacy of vaccines based on CD40 and toll like receptor stimulation has been established, further investigation is needed to understand the mechanism of tumor control and how vaccination alters tumor infiltrating immune cells. In this study we vaccinated mice bearing established murine melanoma tumors with agonistic anti-CD40, polyI:C, and tumor antigen. Vaccination led to increased intratumoral T cell numbers and delayed tumor growth, yet did not require trafficking of T cells from the periphery. Pre-existing intratumoral T cells exhibited an acute burst in proliferation but became less functional in response to vaccination. However, the increased intratumoral T cell numbers yielded increased numbers of effector T cells per tumor. Together, our data indicate that the existing T cell response and intratumoral dendritic cells are critical for vaccination efficacy. It also suggests that circulating T cells responding to vaccination may not be an appropriate biomarker for vaccine efficacy.






Similar content being viewed by others
Data availability
All data relevant to the study are included in the article or uploaded as online supplementary information. Additional information regarding data may be obtained from the authors on reasonable request.
Abbreviations
- BFA:
-
Brefeldin A
- DC:
-
Dendritic cells
- polyI:C:
-
Polyinosinic-polycytidylic acid
- TLR:
-
Toll like receptor
References
Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT et al (2012) Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 72:1070–1080. https://doi.org/10.1158/0008-5472.CAN-11-3218
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571. https://doi.org/10.1038/nature13954
Jäeger E, Bernhard H, Romero P, Ringhoffer M, Arand M, Karbach J et al (1996) Generation of cytotoxic T-cell responses with synthetic melanoma-associated peptides in vivo: implications for tumor vaccines with melanoma-associated antigens. Int J Cancer 66:162–169. https://doi.org/10.1002/(SICI)1097-0215(19960410)66:2%3c162::AID-IJC4%3e3.0.CO;2-0
Cormier JN, Salgaller ML, Prevette T, Barracchini KC, Rivoltini L, Restifo NP et al (1997) Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART-1/Melan A. Cancer J Sci Am 3:37–44
Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL et al (1998) Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 4:321–327. https://doi.org/10.1038/nm0398-321
Slingluff CL, Yamshchikov G, Neese P, Galavotti H, Eastham S, Kittlesen D et al (2001) Phase I trial of a melanoma vaccine with gp100280-288 peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res 7:3012–3024
Marchand M, van Baren N, Weynants P, Brichard V, Dréno B, Tessier M et al (1999) Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer 80:219–230. https://doi.org/10.1002/(SICI)1097-0215(19990118)80:2%3c219::AID-IJC10%3e3.0.CO;2-S
Melssen MM, Petroni GR, Chianese-Bullock KA, Wages NA, Grosh WW, Varhegyi N et al (2019) A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund’s adjuvant in melanoma patients. J Immunother Cancer 7:163. https://doi.org/10.1186/s40425-019-0625-x
Pavlick A, Blazquez AB, Meseck M, Lattanzi M, Ott PA, Marron TU et al (2020) Combined vaccination with NY-ESO-1 protein, poly-ICLC, and montanide improves humoral and cellular immune responses in patients with high-risk melanoma. Cancer Immunol Res 8:70–80. https://doi.org/10.1158/2326-6066.CIR-19-0545
Callan MFC, Tan L, Annels N, Ogg GS, Wilson JDK, O’Callaghan CA et al (1998) Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med 187:1395–1402. https://doi.org/10.1084/jem.187.9.1395
Il CH, Celis E (2009) Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res 69:9012–9019. https://doi.org/10.1158/0008-5472.CAN-09-2019
Schoenberger SP, Toes REM, Van Dervoort EIH, Offringa R, Melief CJM (1998) T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD4OL interactions. Nature 393:480–483. https://doi.org/10.1038/31002
Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A et al (2006) Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol 176:7335–7345. https://doi.org/10.4049/jimmunol.176.12.7335
Salem ML, Kadima AN, Cole DJ, Gillanders WE (2005) Defining the antigen-specific t-cell response to vaccination and poly(I:C)/TLR3 signaling. J Immunother 28:220–228. https://doi.org/10.1097/01.cji.0000156828.75196.0d
Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ et al (2004) Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med 199:775–784. https://doi.org/10.1084/jem.20031591
Llopiz D, Dotor J, Zabaleta A, Lasarte JJ, Prieto J, Borrás-Cuesta F et al (2008) Combined immunization with adjuvant molecules poly(I:C) and anti-CD40 plus a tumor antigen has potent prophylactic and therapeutic antitumor effects. Cancer ImmunolImmunother 57:19–29. https://doi.org/10.1007/s00262-007-0346-8
Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF et al (2013) Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med 19:465–472. https://doi.org/10.1038/nm.3105
Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ et al (2009) Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol 182:5217–5224. https://doi.org/10.4049/jimmunol.0803826
Bialojan A, Sohl J, Rausch J, Aranda Lopez P, Denny M, Langguth P et al (2019) Transcutaneous immunization with CD40 ligation boosts cytotoxic T lymphocyte mediated antitumor immunity independent of CD4 helper cells in mice. Eur J Immunol 49:2083–2094. https://doi.org/10.1002/eji.201848039
Lum HD (2006) In vivo CD40 ligation can induce T cell-independent antitumor effects that involve macrophages. J LeukocBiol 79:1181–1192. https://doi.org/10.1189/jlb.0405191
Medina-Echeverz J, Ma C, Duffy AG, Eggert T, Hawk N, Kleiner DE et al (2015) Systemic agonistic anti-CD40 treatment of tumor-bearing mice modulates hepatic myeloid-suppressive cells and causes immune-mediated liver damage. Cancer Immunol Res 3:557–566. https://doi.org/10.1158/2326-6066.CIR-14-0182
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W et al (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 80-(331):1612–1616. https://doi.org/10.1126/science.1198443
Barrios K, Celis E (2012) TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer ImmunolImmunother 61:1307–1317. https://doi.org/10.1007/s00262-012-1259-8
Hwang ML, Lukens JR, Bullock TNJ (2007) Cognate memory CD4 + T cells generated with dendritic cell priming influence the expansion, trafficking, and differentiation of secondary CD8 + T cells and enhance tumor control. J Immunol 179:5829–5838. https://doi.org/10.4049/jimmunol.179.9.5829
Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ et al (2010) Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8+ T cells. J Immunother 33:769–779. https://doi.org/10.1097/CJI.0b013e3181ee238f
Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R et al (2002) The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J BiolChem 277:21453–21457. https://doi.org/10.1074/jbc.C200176200
Woods AN, Wilson AL, Srivinisan N, Zeng J, Dutta AB, Peske JD et al (2017) Differential expression of homing receptor ligands on tumor-associated vasculature that control CD8 effector T-cell entry. Cancer Immunol Res 5:1062–1073. https://doi.org/10.1158/2326-6066.CIR-17-0190
Rutigliano JA, Johnson TR, Hollinger TN, Fischer JE, Aung S, Graham BS (2004) Treatment with anti-LFA-1 delays the CD8+ cytotoxic-T-lymphocyte response and viral clearance in mice with primary respiratory syncytial virus infection. J Virol 78:3014–3023. https://doi.org/10.1128/jvi.78.6.3014-3023.2004
Setoguchi K, Schenk AD, Ishii D, Hattori Y, Baldwin WM, Tanabe K et al (2011) LFA-1 antagonism inhibits early infiltration of endogenous memory CD8 T cells into cardiac allografts and donor-reactive T cell priming. Am J Transplant 11:923–935. https://doi.org/10.1111/j.1600-6143.2011.03492.x
Pulko V, Liu X, Krco CJ, Harris KJ, Frigola X, Kwon ED et al (2009) TLR3-stimulated dendritic cells up-regulate B7–H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination. J Immunol 183:3634–3641. https://doi.org/10.4049/jimmunol.0900974
Azuma M, Takeda Y, Nakajima H, Sugiyama H, Ebihara T, Oshiumi H et al (2016) Biphasic function of TLR3 adjuvant on tumor and spleen dendritic cells promotes tumor T cell infiltration and regression in a vaccine therapy. Oncoimmunology 5:e1188244. https://doi.org/10.1080/2162402X.2016.1188244
Gemta LF, Siska PJ, Nelson ME, Gao X, Liu X, Locasale JW et al (2019) Impaired enolase 1 glycolytic activity restrains effector functions of tumor-infiltrating CD8+ T cells. SciImmunol. https://doi.org/10.1126/sciimmunol.aap9520
Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE et al (2012) Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 80-(338):1220–1225. https://doi.org/10.1126/science.1229620
Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC et al (2016) Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537:417–421. https://doi.org/10.1038/nature19330
Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D et al (2019) Intratumoral Tcf1 + PD-1 + CD8 + T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50(195–211):e10. https://doi.org/10.1016/j.immuni.2018.12.021
Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA et al (2007) Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J ClinOncol 25:876–883. https://doi.org/10.1200/JCO.2006.08.3311
Sanborn RE, Gabrail NY, Bhardwaj N, Gordon MS, O’Hara M, Khalil D et al (2019) Abstract LB-194: first-in-human phase I study of the CD40 agonist mAb CDX-1140 and in combination with CDX-301 (rhFLT3L) in patients with advanced cancers: Interim results. Cancer Res Am Assoc Cancer Res (AACR). https://doi.org/10.1158/1538-7445.am2019-lb-194 (LB-194-LB-194)
Grilley-Olson JE, Curti BD, Smith DC, Goel S, Gajewski T, Markovic S et al (2018) SEA-CD40, a non-fucosylated CD40 agonist: interim results from a phase 1 study in advanced solid tumors. J ClinOncol 36:3093–3093. https://doi.org/10.1200/jco.2018.36.15_suppl.3093
Vonderheide RH, Burg JM, Mick R, Trosko JA, Li D, Shaik MN et al (2013) Phase i study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology. https://doi.org/10.4161/onci.23033
Bajor DL, Mick R, Riese MJ, Huang AC, Sullivan B, Richman LP et al (2018) Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology. https://doi.org/10.1080/2162402X.2018.1468956
Acknowledgements
The authors would like to thank Dr. Alexandra Witter and Ms. Marissa Gonzales for assistance and discussions and the Beirne B. Carter Center for Immunology Research for use of the flow cytometry instruments.
Funding
This work was funded by National Institutes of Health grant R01CA166458 to Timothy N.J. Bullock. Aaron D. Stevens was supported by National Institutes of Health training Grant T32AI007496.
Author information
Authors and Affiliations
Contributions
All authors conceived the project, designed experiments, interpreted data, and wrote the manuscript. A.D.S. performed experiments and analyzed data. T.N.J.B. supervised and acquired funding.
Corresponding author
Ethics declarations
Conflict of interests
None declared.
Ethics approval
All mice were treated in accordance with policies established by the University of Virginia Animal Care and Use Committee (protocol 3292).
Consent for publication
All authors have reviewed and approved the submitted manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stevens, A.D., Bullock, T.N.J. Therapeutic vaccination targeting CD40 and TLR3 controls melanoma growth through existing intratumoral CD8 T cells without new T cell infiltration. Cancer Immunol Immunother 70, 2139–2150 (2021). https://doi.org/10.1007/s00262-020-02841-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02841-z