Abstract
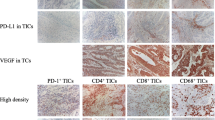
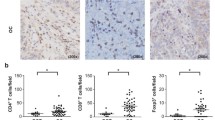
Due to its high ability to disseminate, ovarian cancer remains one of the largest threats to women’s health, worldwide. Evidence showed that the immune cells infiltrating the tumor microenvironment are crucial in mediating metastasis. Therefore, it is necessary to understand which types of immune cells are involved in metastasis, and to determine the mechanisms by which they influence the process. By immunohistochemistry, we found that higher concentrations of intratumoral CD8+ T cells were found to be correlated with an advanced grade and stage of ovarian cancer. Additionally, the infiltration of stromal CD8+ T cells was also significantly higher in tissues with advanced stages and metastatic tumors. A positive correlation between the infiltration of FoxP3+ Treg cells and histological grade was also observed, regardless of location. PD-L1 expression in metastatic tumors was also higher than that in paired primary ovarian tumors. Transwell migration and invasion assays revealed the increased migration and invasion of ovarian cancer cell lines (A2780CP and ES2) and ascites-derived ovarian cancer cells following co-culturing with CD8+ T cells. Enhanced expression of MMP-9, uPA, VEGF, bFGF, IL-8, IL-10, and PD-L1 by cancer cells following co-culturing with CD8+ T cells were also detected by qPCR, ELISA or flow cytometry. In conclusion, our findings suggest that the infiltrated T cells could promote the development of ovarian cancer, and provide another mechanism of immune evasion mediated by T cells.







Similar content being viewed by others
References
American Cancer Society (2011) Cancer Facts & Figures 2011. Atlanta: American Cancer Society
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL (2018) Ovarian cancer statistics. CA Cancer J Clin 68(4):284–296. https://doi.org/10.3322/caac.21456
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. https://doi.org/10.1016/j.cell.2011.02.013
Kulbe H, Chakravarty P, Leinster DA, Charles KA, Kwong J, Thompson RG, Coward JI, Schioppa T, Robinson SC, Gallagher WM, Galletta L, Australian Ovarian Cancer Study G, Salako MA, Smyth JF, Hagemann T, Brennan DJ, Bowtell DD, Balkwill FR (2012) A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Can Res 72(1):66–75. https://doi.org/10.1158/0008-5472.CAN-11-2178
Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schworer AM, Wagner U, Muller-Brusselbach S, Muller R (2014) Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer J Int Du Cancer 134(1):32–42. https://doi.org/10.1002/ijc.28335
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124(2):263–266. https://doi.org/10.1016/j.cell.2006.01.007
Drakes ML, Stiff PJ (2018) Regulation of ovarian cancer prognosis by immune cells in the tumor microenvironment. Cancers (Basel). https://doi.org/10.3390/cancers10090302
Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ (2003) CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol 171(11):5931–5939
Maloy KJ, Powrie F (2001) Regulatory T cells in the control of immune pathology. Nat Immunol 2(9):816–822. https://doi.org/10.1038/ni0901-816
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102(51):18538–18543. https://doi.org/10.1073/pnas.0509182102
Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C (2005) The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 11(23):8326–8331. https://doi.org/10.1158/1078-0432.CCR-05-1244
Shepherd TG, Theriault BL, Campbell EJ (2007) Nachtigal MW (2015) Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients (vol 1, pg 2643, 2007). Nat Protoc 10(9):1457–1457. https://doi.org/10.1038/nprot0915-1457b
Wang J-J, Siu MK, Jiang Y-X, Leung TH, Chan DW, Cheng R-R, Cheung AN, Ngan HY, Chan KK (2019) Aberrant upregulation of PDK1 in ovarian cancer cells impairs CD8+ T cell function and survival through elevation of PD-L1. OncoImmunology 8(11):1659092. https://doi.org/10.1080/2162402X.2019.1659092
Shi J, Wei PK (2015) Low-dose interleukin-8 induces the adhesion, migration and invasion of the gastric cancer SGC-7901 cell line. Oncol Lett 10(5):2871–2877. https://doi.org/10.3892/ol.2015.3641
Mustea A, Konsgen D, Braicu EI, Pirvulescu C, Sun P, Sofroni D, Lichtenegger W, Sehouli J (2006) Expression of IL-10 in patients with ovarian carcinoma. Anticancer Res 26(2C):1715–1718
Lane D, Matte I, Garde-Granger P, Bessette P, Piche A (2018) Ascites IL-10 promotes ovarian cancer cell migration. Cancer Microenviron 11(2–3):115–124. https://doi.org/10.1007/s12307-018-0215-3
Pietzner K, Nasser S, Alavi S, Darb-Esfahani S, Passler M, Muallem MZ, Sehouli J (2018) Checkpoint-inhibition in ovarian cancer: rising star or just a dream? J Gynecol Oncol 29(6):e93. https://doi.org/10.3802/jgo.2018.29.e93
Wang S, Li J, Xie J, Liu F, Duan Y, Wu Y, Huang S, He X, Wang Z, Wu X (2018) Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin beta4/SNAI1/SIRT3 signaling pathway. Oncogene 37(30):4164–4180. https://doi.org/10.1038/s41388-018-0252-x
Li J, Chen L, Xiong Y, Zheng X, Xie Q, Zhou Q, Shi L, Wu C, Jiang J, Wang H (2017) Knockdown of PD-L1 in human gastric cancer cells inhibits tumor progression and improves the cytotoxic sensitivity to CIK therapy. Cell Physiol Biochem 41(3):907–920. https://doi.org/10.1159/000460504
Yahata T, Mizoguchi M, Kimura A, Orimo T, Toujima S, Kuninaka Y, Nosaka M, Ishida Y, Sasaki I, Fukuda-Ohta Y, Hemmi H, Iwahashi N, Noguchi T, Kaisho T, Kondo T, Ino K (2019) Programmed cell death ligand 1 disruption by clustered regularly interspaced short palindromic repeats/Cas9-genome editing promotes antitumor immunity and suppresses ovarian cancer progression. Cancer Sci 110(4):1279–1292. https://doi.org/10.1111/cas.13958
Christofori G (2006) New signals from the invasive front. Nature 441(7092):444–450. https://doi.org/10.1038/nature04872
Torre LA, Islami F, Siegel RL, Ward EM, Jemal A (2017) global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev 26(4):444–457. https://doi.org/10.1158/1055-9965.EPI-16-0858
Moffitt L, Karimnia N, Stephens A, Bilandzic M (2019) Therapeutic targeting of collective invasion in ovarian cancer. Int J Mol Sci. https://doi.org/10.3390/ijms20061466
Yasukawa M, Liu Y, Hu L, Cogdell D, Gharpure KM, Pradeep S, Nagaraja AS, Sood AK, Zhang W (2017) ADAMTS16 mutations sensitize ovarian cancer cells to platinum-based chemotherapy. Oncotarget 8(51):88410–88420. https://doi.org/10.18632/oncotarget.11120
Blomberg OS, Spagnuolo L, de Visser KE (2018) Immune regulation of metastasis: mechanistic insights and therapeutic opportunities. Dis Model Mech. https://doi.org/10.1242/dmm.036236
Yaguchi T, Sumimoto H, Kudo-Saito C, Tsukamoto N, Ueda R, Iwata-Kajihara T, Nishio H, Kawamura N, Kawakami Y (2011) The mechanisms of cancer immunoescape and development of overcoming strategies. Int J Hematol 93(3):294–300. https://doi.org/10.1007/s12185-011-0799-6
Meng Y, Liang H, Hu J, Liu S, Hao X, Wong MSK, Li X, Hu L (2018) PD-L1 expression correlates with tumor infiltrating lymphocytes and response to neoadjuvant chemotherapy in cervical cancer. J Cancer 9(16):2938–2945. https://doi.org/10.7150/jca.22532
Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, Yang Y, Wang L, Cao X, Wang J (2012) Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol 188(12):5954–5961. https://doi.org/10.4049/jimmunol.1103466
Nyberg P, Xie L, Kalluri R (2005) Endogenous inhibitors of angiogenesis. Can Res 65(10):3967–3979. https://doi.org/10.1158/0008-5472.CAN-04-2427
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362(6423):841–844. https://doi.org/10.1038/362841a0
Guo H, Zhu Q, Yu X, Merugu SB, Mangukiya HB, Smith N, Li Z, Zhang B, Negi H, Rong R, Cheng K, Wu Z, Li D (2017) Tumor-secreted anterior gradient-2 binds to VEGF and FGF2 and enhances their activities by promoting their homodimerization. Oncogene 36(36):5098–5109. https://doi.org/10.1038/onc.2017.132
Khochenkov DA, Solomko ES, Peretolchina NM, Ryabaya OO, Stepanova EV (2015) Antiangiogenic activity of alofanib, an allosteric inhibitor of fibroblast growth factor receptor 2. Bull Exp Biol Med 160(1):84–87. https://doi.org/10.1007/s10517-015-3104-5
Zamarron BF, Chen W (2011) Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 7(5):651–658
Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14(21):6735–6741. https://doi.org/10.1158/1078-0432.CCR-07-4843
Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, Dinney CP (2000) Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res 6(5):2104–2119
Zhou J, Ye F, Chen H, Lv W, Gan N (2007) The expression of interleukin-10 in patients with primary ovarian epithelial carcinoma and in ovarian carcinoma cell lines. J Int Med Res 35(3):290–300. https://doi.org/10.1177/147323000703500302
Sheikhpour E, Noorbakhsh P, Foroughi E, Farahnak S, Nasiri R, Neamatzadeh H (2018) A survey on the role of interleukin-10 in breast cancer: a narrative. Rep Biochem Mol Biol 7(1):30–37
Kim S, Lee J, Jeon M, Lee JE, Nam SJ (2016) MEK-dependent IL-8 induction regulates the invasiveness of triple-negative breast cancer cells. Tumour Biol 37(4):4991–4999. https://doi.org/10.1007/s13277-015-4345-7
Qiu XY, Hu DX, Chen WQ, Chen RQ, Qian SR, Li CY, Li YJ, Xiong XX, Liu D, Pan F, Yu SB (1864) Chen XQ (2018) PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim Biophys Acta Mol Basis Dis 5:1754–1769. https://doi.org/10.1016/j.bbadis.2018.03.002
Wang Y, Wang H, Zhao Q, Xia Y, Hu X, Guo J (2015) PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med Oncol 32(8):212. https://doi.org/10.1007/s12032-015-0655-2
Liao Y, Chen L, Feng Y, Shen J, Gao Y, Cote G, Choy E, Harmon D, Mankin H, Hornicek F, Duan Z (2017) Targeting programmed cell death ligand 1 by CRISPR/Cas9 in osteosarcoma cells. Oncotarget 8(18):30276–30287. https://doi.org/10.18632/oncotarget.16326
Qu QX, Xie F, Huang Q, Zhang XG (2017) Membranous and cytoplasmic expression of PD-L1 in ovarian cancer cells. Cell Physiol Biochem 43(5):1893–1906. https://doi.org/10.1159/000484109
Mailloux AW, Young MR (2010) Regulatory T-cell trafficking: from thymic development to tumor-induced immune suppression. Crit Rev Immunol 30(5):435–447
Shi C, Chen Y, Chen Y, Yang Y, Bing W, Qi J (2019) CD4(+) CD25(+) regulatory T cells promote hepatocellular carcinoma invasion via TGF-beta1-induced epithelial-mesenchymal transition. Onco Targets Ther 12:279–289. https://doi.org/10.2147/OTT.S172417
Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y, Wang HY, Cheng SQ, Xie D, Wang XF (2012) TGF-beta-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell 22(3):291–303. https://doi.org/10.1016/j.ccr.2012.07.023
Barnett JC, Bean SM, Whitaker RS, Kondoh E, Baba T, Fujii S, Marks JR, Dressman HK, Murphy SK, Berchuck A (2010) Ovarian cancer tumor infiltrating T-regulatory (T(reg)) cells are associated with a metastatic phenotype. Gynecol Oncol 116(3):556–562. https://doi.org/10.1016/j.ygyno.2009.11.020
Funding
The work was funded by the Research Fund from the Department of Obstetrics and Gynaecology, the University of Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Ethics approval
Sample collection was under the approval of Human Research Ethics Committee of the University of Hong Kong.
Consent to participate
Consented patients were informed of the use of their clinical information before sample collection. The authors confirm that they have obtained written consent from each patient to publish the manuscript.
Consent for publication
This manuscript does not contain any individual person’s data.
Availability of data and material
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, JJ., Siu, M.KY., Jiang, YX. et al. Infiltration of T cells promotes the metastasis of ovarian cancer cells via the modulation of metastasis-related genes and PD-L1 expression. Cancer Immunol Immunother 69, 2275–2289 (2020). https://doi.org/10.1007/s00262-020-02621-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02621-9