Abstract
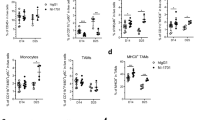
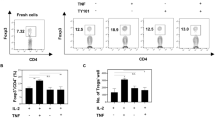
A major factor impeding the success of numerous therapeutic approaches in cancer is the immunosuppressive nature of the tumor microenvironment (TME). Hence, methods capable of reverting tumor immunosuppression through depletion or reprogramming of myeloid-derived suppressive cells (MDSCs) and regulatory T cells (Tregs) are of great clinical need. Here, we explore NKG2D-Fc as a modality to modulate antitumor immunity through the depletion of immunosuppressive MDSCs and Tregs in the TME. We have generated the NKG2D-Fc fusion protein and characterized its potential to mediate tumor control and overall survival in LL2 and MC38 murine models. Upon treatment of LL2 or MC38 tumor-bearing mice with NKG2D-Fc, we observe significant tumor control and enhanced survival compared to Fc control. When characterizing MDCSs and Tregs from tumor-bearing mice, we observe clear expression of NKG2D-ligand RAE1γ and subsequent binding of NKG2D-Fc fusion protein to both MDSCs and Tregs. Examining the immune profile of mice treated with NKG2D-Fc reveals significant depletion of MDSCs and Tregs in the TME, as well as an increase in NK cells likely due to the reversed suppressive TME. In conclusion, NKG2D-Fc induces antitumor immunity and tumor control through the depletion of MDSCs and Tregs, subsequently providing a niche for the infiltration and expansion of proinflammatory cells, such as NK cells. Strategies capable of modulating the immunosuppressive state in cancer are in high clinical demand. NKG2D-Fc is a simple, single tool capable of depleting both MDSCs and Tregs and should be further investigated as a therapeutic agent for the treatment of cancer.




Similar content being viewed by others
Availability of data and material
All data recorded for this study was performed at the Johns Hopkins Medical Institutes.
Abbreviations
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- ARG1:
-
Arginase 1
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- ICB:
-
Immune checkpoint blockade
- ICOS:
-
Inducible T cell costimulatory
- IFNγ:
-
Interferon gamma
- IL-10:
-
Interleukin-10
- MDSCs:
-
Myeloid-derived suppressor cells
- MHC:
-
Major histocompatibility complex
- M-MDSCs:
-
Monocytic MDSCs
- NO:
-
Nitric oxide
- PD-L1:
-
Program death-ligand 1
- PGE2:
-
Prostaglandin E2
- PMN-MDSCs:
-
Granulocytic MDSCs
- ROS:
-
Reactive oxidative species
- scFv-NKG2D:
-
Single-chain variable fragment with the extracellular domain of NKG2D receptor
- TAMs:
-
Tumor-associated macrophages
- TGF-β:
-
Transforming growth factor beta
- Tregs:
-
Regulatory T cells
References
Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22:329–360. https://doi.org/10.1146/annurev.immunol.22.012703.104803
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3:991–998. https://doi.org/10.1038/ni1102-991
de Charette M, Houot R (2018) Hide or defend, the two strategies of lymphoma immune evasion: potential implications for immunotherapy. Haematologica 103:1256–1268. https://doi.org/10.3324/haematol.2017.184192
Lin YC, Mahalingam J, Chiang JM et al (2013) Activated but not resting regulatory T cells accumulated in tumor microenvironment and correlated with tumor progression in patients with colorectal cancer. Int J Cancer 132:1341–1350. https://doi.org/10.1002/ijc.27784
Beyer M, Schultze JL (2006) Regulatory T cells in cancer. Blood 108:804–811. https://doi.org/10.1182/blood-2006-02-002774
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V (2012) Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12:253–268. https://doi.org/10.1038/nri3175
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307. https://doi.org/10.1038/nri1806
Feng PH, Lee KY, Chang YL et al (2012) CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med 186:1025–1036. https://doi.org/10.1164/rccm.201204-0636OC
Liu CY, Wang YM, Wang CL et al (2010) Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b +/CD14(-)/CD15 +/CD33 + myeloid-derived suppressor cells and CD8 + T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol 136:35–45. https://doi.org/10.1007/s00432-009-0634-0
Mao Y, Poschke I, Wennerberg E et al (2013) Melanoma-educated CD14 + cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res 73:3877–3887. https://doi.org/10.1158/0008-5472.CAN-12-4115
Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC (2005) Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med 202:931–939. https://doi.org/10.1084/jem.20050715
Scurr M, Ladell K, Besneux M et al (2014) Highly prevalent colorectal cancer-infiltrating LAP(+) Foxp3(−) T cells exhibit more potent immunosuppressive activity than Foxp3(+) regulatory T cells. Mucosal Immunol 7:428–439. https://doi.org/10.1038/mi.2013.62
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355. https://doi.org/10.1126/science.aar4060
Srivastava MK, Zhu L, Harris-White M et al (2012) Targeting myeloid-derived suppressor cells augments antitumor activity against lung cancer. Immunotargets Ther. 2012:7–12. https://doi.org/10.2147/ITT.S32617
Golgher D, Jones E, Powrie F, Elliott T, Gallimore A (2002) Depletion of CD25 + regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol 32:3267–3275. https://doi.org/10.1002/1521-4141(200211)32:11%3c3267:AID-IMMU3267%3e3.0.CO;2-1
Zhang T, Sentman CL (2011) Cancer immunotherapy using a bispecific NK receptor fusion protein that engages both T cells and tumor cells. Cancer Res 71:2066–2076. https://doi.org/10.1158/0008-5472.CAN-10-3200
Kang TH, Mao CP, He L, Tsai YC, Liu K, La V, Wu TC, Hung CF (2012) Tumor-targeted delivery of IL-2 by NKG2D leads to accumulation of antigen-specific CD8 + T cells in the tumor loci and enhanced anti-tumor effects. PLoS ONE 7:e35141. https://doi.org/10.1371/journal.pone.0035141
Hu J, Bernatchez C, Zhang L, Xia X, Kleinerman ES, Hung MC, Hwu P, Li S (2017) Induction of NKG2D ligands on solid tumors requires tumor-specific CD8(+) T cells and histone acetyltransferases. Cancer Immunol Res 5:300–311. https://doi.org/10.1158/2326-6066.CIR-16-0234
Jung H, Hsiung B, Pestal K, Procyk E, Raulet DH (2012) RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J Exp Med 209:2409–2422. https://doi.org/10.1084/jem.20120565
Park YJ, Kuen DS, Chung Y (2018) Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med 50:109. https://doi.org/10.1038/s12276-018-0130-1
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809. https://doi.org/10.1038/nrc2734
Lesokhin AM, Hohl TM, Kitano S et al (2012) Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res 72:876–886. https://doi.org/10.1158/0008-5472.CAN-11-1792
Umansky V, Blattner C, Gebhardt C, Utikal J (2017) CCR5 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunol Immunother 66:1015–1023. https://doi.org/10.1007/s00262-017-1988-9
Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, Balkwill FR (2003) A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res 63:8360–8365
Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, Utikal J, Umansky V (2018) Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front Immunol 9:398. https://doi.org/10.3389/fimmu.2018.00398
Romano E, Kusio-Kobialka M, Foukas PG et al (2015) Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci USA 112:6140–6145. https://doi.org/10.1073/pnas.1417320112
Callahan MK, Postow MA, Wolchok JD (2014) CTLA-4 and PD-1 Pathway Blockade: combinations in the Clinic. Front Oncol 4:385. https://doi.org/10.3389/fonc.2014.00385
Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH (2000) Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol 1:119–126. https://doi.org/10.1038/77793
Ghiringhelli F, Menard C, Terme M et al (2005) CD4 + CD25 + regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 202:1075–1085. https://doi.org/10.1084/jem.20051511
Acknowledgements
The authors would like to acknowledge Dr. T.-C. Wu for facilitating helpful science discussions and Mr. Christopher Carter Polston for providing administrative assistance. This study was supported by an NIH-supported research grant (R01CA233486).
Author information
Authors and Affiliations
Contributions
PHF and BL equally contributed to the design of the study, the performance of the experiments, and the writing of the manuscript; SHT and YJK contributed to the performance of the experiments; EF and MC contributed to the writing and preparation of the manuscript; CH contributed to the design of the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not declare any competing interests.
Ethics approval and consent to participate
The housing and handling of mice follow guidelines established by Johns Hopkins Medical Institutions Animal Care and Use Committee and the National Institutes of Health. The Oncology Center Animal Facility has full-time veterinary support through the Department of Comparative Medicine. Animals are monitored daily for infection and other illnesses by trained animal technicians. The criteria include: infection, failure to thrive, perceived pain, respiratory distress, etc. Research support services include training classes and the capacity for full post-mortem analysis is available on request. Only trained laboratory personnel and animal technicians were allowed to handle laboratory animals. All individuals handling mice were registered to protocols at the Johns Hopkins Animal Care and Use Committee.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig.
1. Anti-tumor effects of NKG2D-Fc at a lower dose. (A) MC38 tumor-bearing mice were treated with 30 μg of NKG2D-Fc or Fc control protein. Same kinetic was followed as in Fig. 1. Tumor growth and Kaplan–Meier survival curves were collected. Data are represented as mean ± SEM, with n = 5 in each group from one representative experiment (PNG 14 kb)
Supplementary Fig.
2. Gating strategy for Tregs and MDSCs. (A) Representative flow dot plots from spleen depicting gating strategy used to identify MDSC and Tregs in PBMC, spleen, and tumor of mice (PNG 336 kb)
Supplementary Fig.
3. RAE1 γ staining on circulating MDSCs and Tregs in naïve or tumor-bearing mice. (A) Representative histogram of RAE1 γ on MDSCs and (B) MFI represented as mean ± SEM. (C) Representative histogram of RAE1 γ on Tregs and (B) MFI represented as mean ± SEM. Naïve mice are shown as a grey histogram, and tumor-bearing mice as a purple histogram (PNG 98 kb)
Rights and permissions
About this article
Cite this article
Feng, PH., Lam, B., Tseng, SH. et al. NKG2D-Fc fusion protein promotes antitumor immunity through the depletion of immunosuppressive cells. Cancer Immunol Immunother 69, 2147–2155 (2020). https://doi.org/10.1007/s00262-020-02615-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02615-7