Abstract
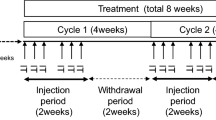
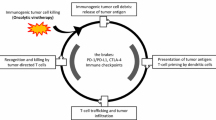
Hemagglutinating virus of Japan (HVJ; Sendai virus) is an RNA virus that has cell fusion activity. HVJ-envelope (HVJ-E) is a UV-irradiated HVJ particle that loses viral replication and protein synthesis activity but retains cell fusion activity. We recently reported that HVJ-E has antitumor effects on several types of tumors. Here, we describe the results of a first-in-human phase I/IIa study in patients with advanced melanoma, receiving intratumoral administration of HVJ-E. The primary aim was to evaluate the safety and tolerability of HVJ-E, and the secondary aim was to examine the objective tumor response and antitumor immunity. Six patients with stage IIIC or IV progressive malignant melanoma with skin or lymph metastasis were enrolled. Patients were separated into two groups (n = 3 each) and received low and high doses of HVJ-E. Five of the six patients completed 4 weeks of follow-up evaluation; one patient discontinued treatment owing to progressive disease. Complete or partial responses were observed in 3 of 6 (50%) injected target lesions, 7 of 15 (47%) noninjected target lesions, and 10 of 21 (48%) target lesions. Induction of antitumor immunity was observed: activation of natural killer cells, a marked increase in interferon-γ levels in the peripheral blood, and infiltration of cytotoxic T cells into both injected and noninjected tumor lesions. Thus, intratumoral injection of HVJ-E in advanced melanoma patients showed safety and tolerability with local regression of the tumor mediated by antitumor immunity. The results suggest that HVJ-E might be a new treatment approach in patients with advanced melanoma.



Similar content being viewed by others
Abbreviations
- CR:
-
Complete response
- DLT:
-
Dose-limiting toxicity
- ECOG:
-
Eastern Cooperative Oncology Group
- HVJ:
-
Hemagglutinating virus of Japan
- HVJ-E:
-
Inactivated HVJ-envelope
- mNAU:
-
Milli neuraminidase unit
- MTD:
-
Maximum tolerated dose
- NCI-CTCAE:
-
National Cancer Institute Common Toxicity Criteria for Adverse Events
- PD:
-
Progressive disease
- PR:
-
Partial response
- RIG-I:
-
Retinoic acid-inducible gene-I
- SD:
-
Stable disease
References
Okada Y (1993) Sendai virus-induced cell fusion. Methods Enzymol 221:18–41
Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N, Nakamura H, Morishita R, Kotani H (2002) Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther 6:219–226
Kondo Y, Fushikida K, Fujieda T, Sakai K, Miyata K, Kato F, Kato M (2008) Efficient delivery of antibody into living cells using a novel HVJ envelope vector system. J Immunol Methods 332:10–17. https://doi.org/10.1016/j.jim.2007.12.008
Kurooka M, Kaneda Y (2007) Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res 67:227–236. https://doi.org/10.1158/0008-5472.CAN-06-1615
Fujihara A, Kurooka M, Miki T, Kaneda Y (2008) Intratumoral injection of inactivated Sendai virus particles elicits strong antitumor activity by enhancing local CXCL10 expression and systemic NK cell activation. Cancer Immunol Immunother 57:73–84. https://doi.org/10.1007/s00262-007-0351-y
Kawaguchi Y, Miyamoto Y, Inoue T, Kaneda Y (2009) Efficient eradication of hormone-resistant human prostate cancers by inactivated Sendai virus particle. Int J Cancer 124:2478–2487. https://doi.org/10.1002/ijc.24234
Matsuda M, Nimura K, Shimbo T, Hamasaki T, Yamamoto T, Matsumura A, Kaneda Y (2011) Immunogene therapy using immunomodulating HVJ-E vector augments anti-tumor effects in murine malignant glioma. J Neurooncol 103:19–31. https://doi.org/10.1007/s11060-010-0355-x
Matsushima-Miyagi T, Hatano K, Nomura M, Li-Wen L, Nishikawa T, Saga K, Shimbo T, Kaneda Y (2012) TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of the RIG-I/MAVS signaling pathway by nonreplicating Sendai virus particles. Clin Cancer Res 18:6271–6283. https://doi.org/10.1158/1078-0432.CCR-12-1595
Robert C, Long GV, Brady B et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330. https://doi.org/10.1056/NEJMoa1412082
Hodi FS, O'Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. https://doi.org/10.1056/NEJMoa1003466
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133. https://doi.org/10.1056/NEJMoa1302369
Morton DL, Eilber FR, Holmes EC, Hunt JS, Ketcham AS, Silverstein MJ, Sparks FC (1974) BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg 180:635–643
Hwang TH, Moon A, Burke J et al (2011) A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther 19:1913–1922. https://doi.org/10.1038/mt.2011.132
Kaufman HL, Deraffele G, Mitcham J et al (2005) Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J Clin Investig 115:1903–1912. https://doi.org/10.1172/JCI24624
Senzer NN, Kaufman HL, Amatruda T et al (2009) Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 27:5763–5771. https://doi.org/10.1200/JCO.2009.24.3675
Kiyohara E, Tamai K, Katayama I, Kaneda Y (2012) The combination of chemotherapy with HVJ-E containing Rad51 siRNA elicited diverse anti-tumor effects and synergistically suppressed melanoma. Gene Ther 19:734–741. https://doi.org/10.1038/gt.2011.123
Tanemura A, Kiyohara E, Katayama I, Kaneda Y (2013) Recent advances and developments in the antitumor effect of the HVJ envelope vector on malignant melanoma: from the bench to clinical application. Cancer Gene Ther 20:599–605. https://doi.org/10.1038/cgt.2013.61
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Green S, Benedetti J, Crowley J (2002) Clinical trials in oncology, 2nd edn. CRC Press, UK
Nakajima T, Itai T, Wada H, Yamauchi T, Kiyohara E, Kaneda Y (2013) A novel therapy for melanoma and prostate cancer using a non-replicating Sendai virus particle (HVJ-E). IntechOpen. https://doi.org/10.5772/55014
National Cancer Institute NIoH, US Department of Health and Human Services (2008) Common terminology criteria for adverse events (CTCAE), ver 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
Hu JC, Coffin RS, Davis CJ et al (2006) A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 12:6737–6747. https://doi.org/10.1158/1078-0432.CCR-06-0759
Weide B, Martens A, Wistuba-Hamprecht K et al (2017) Combined treatment with ipilimumab and intratumoral interleukin-2 in pretreated patients with stage IV melanoma-safety and efficacy in a phase II study. Cancer Immunol Immunother 66:441–449. https://doi.org/10.1007/s00262-016-1944-0
Seya T, Azuma M, Matsumoto M (2013) Targeting TLR3 with no RIG-I/MDA5 activation is effective in immunotherapy for cancer. Expert Opin Ther Targets 17:533–544. https://doi.org/10.1517/14728222.2013.765407
Fujita K, Nakai Y, Kawashima A et al (2017) Phase I/II clinical trial to assess safety and efficacy of intratumoral and subcutaneous injection of HVJ-E in castration-resistant prostate cancer patients. Cancer Gene Ther 24:277–281. https://doi.org/10.1038/cgt.2017.15
Danielli R, Patuzzo R, Di Giacomo AM et al (2015) Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother 64:999–1009. https://doi.org/10.1007/s00262-015-1704-6
Thompson JF, Hersey P, Wachter E (2008) Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res 18:405–411. https://doi.org/10.1097/CMR.0b013e32831328c7
Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S (2010) Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 17:718–730. https://doi.org/10.1245/s10434-009-0809-6
Quaglino P, Marenco F, Osella-Abate S, Cappello N, Ortoncelli M, Salomone B, Fierro MT, Savoia P, Bernengo MG (2010) Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol 21:409–414. https://doi.org/10.1093/annonc/mdp325
Rozera C, Cappellini GA, D'Agostino G et al (2015) Intratumoral injection of IFN-alpha dendritic cells after dacarbazine activates anti-tumor immunity: results from a phase I trial in advanced melanoma. J Transl Med 13:139. https://doi.org/10.1186/s12967-015-0473-5
Kaufman HL, Amatruda T, Reid T et al (2016) Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J Immunother Cancer 4:12. https://doi.org/10.1186/s40425-016-0116-2
Puzanov I, Milhem MM, Minor D et al (2016) Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol 34:2619–2626. https://doi.org/10.1200/JCO.2016.67.1529
Ribas A, Dummer R, Puzanov I et al (2017) Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170(1109–1119):e1110. https://doi.org/10.1016/j.cell.2017.08.027
Tumeh PC, Harview CL, Yearley JH et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571. https://doi.org/10.1038/nature13954
Acknowledgements
We thank the patients, study staff, and investigators in this study. We also thank Eriko Nobuyoshi for the support in immunostaining. We would like to thank Editage (https://www.editage.com) for English language editing.
Funding
This study was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation.
Author information
Authors and Affiliations
Contributions
Study conception and design: EK, AT, KS, TN, YK, and IK. Patient recruitment and management: EK, AT, MN, MY, AT, AY, and IK. Data acquisition, management, analysis and interpretation: EK, AT, AS, KS, AM, TS, YK, and IK. Manuscript preparation: EK, AT, TN, YK, and IK. All authors read and approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
Toshihiro Nakajima is the employee of GenomIdea, Inc. Toshihiro Nakajima and Yasufumi Kaneda are stock-holders (0.08 and 0.5%) of GenomIdea, Inc. The other authors declare no conflict of interest.
Ethical approval and ethical standards
This study was performed with the approval of the ethics committee at Osaka University Hospital (No. 746 on July 28th 2009). The project was allocated at the Medical Center for Translational Research with number MM0901. All procedures involving human participants were carried out in accordance with the ethical standards of the Osaka University Hospital, national research committee (the University Hospital Medical Information Network-UMIN000002376 and UMIN000012943) and in line with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent for protocol therapy, analysis of patient samples, and use of data for research and publication were obtained from all individual participants prior to treatment in the study.
Cell line authentication
The wild-type HVJ (Z strain) was produced using HEK293 cells, which were purchased from the Japanese Collection of Research Bioresources Cell Bank (JCRB9068, Japan) and cloned as a GenomIdea Clone 20 (GIC20) to prepare the master cell bank for manufacture. Isozyme analysis was performed for authenticating the cell line. Four types of isozymes (lactate dehydrogenase, glucose-6-phosphate dehydrogenase, malate dehydrogenase, and nucleoside phosphorylase) of the master cell bank were analyzed for the identification and characterization of the cell line in GLP study at BioReliance (Stirling, Scotland).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiyohara, E., Tanemura, A., Nishioka, M. et al. Intratumoral injection of hemagglutinating virus of Japan-envelope vector yielded an antitumor effect for advanced melanoma: a phase I/IIa clinical study. Cancer Immunol Immunother 69, 1131–1140 (2020). https://doi.org/10.1007/s00262-020-02509-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02509-8