Abstract
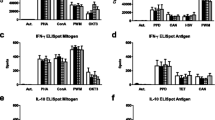
The radiolabeled somatostatin analogue, yttrium-90 DOTA-d-Phe(1)-Tyr(3)-octreotide (DOTATOC), is currently applied to treat advanced somatostatin receptor-positive tumors, e.g., neuroendocrine tumors of the pancreas, lung or gut. However, effects of this treatment on antimicrobial immune responses are not yet defined. In 20 patients treated with DOTATOC, cellular in vitro immune function was determined. Their antimicrobial lymphocyte responses were assessed by lymphocyte transformation test and enzyme-linked immunospot—measuring lymphocyte proliferation and on a single cell level production of pro- and anti-inflammatory cytokines (interferon-γ and interleukin-10)—prior to therapy, at day 1, day 7 and day 90 post-therapy. Proliferative lymphocyte responses and interferon-γ production after in vitro stimulation with microbial antigens were non-significantly suppressed at day 1 and significantly (p < 0.05) at day 7 versus pre-therapy. In vitro immune responses did not fully recover until day 90. In contrast, at day 1 interleukin-10 production was significantly (p < 0.05) increased. Taken together, we observed a decrease in pro-inflammatory immune responses after DOTATOC therapy. Patients with versus without bone metastases displayed significantly (p < 0.05) lower cellular immune responses toward several microbial antigens. Progressive disease and higher tumor burden could also be defined as factors associated with impaired immune function. Spearman correlation analysis indicated that cellular in vitro immunity was positively correlated with kidney function; better kidney function led to stronger immune responses. In conclusion, DOTATOC therapy caused a decrease in in vitro immune responses against microorganisms. The clinical impact needs to be evaluated in further studies.




Similar content being viewed by others
Abbreviations
- Cpm:
-
Counts per minute
- DOTATOC:
-
DOTA-d-Phe(1)-Tyr(3)-octreotide
- DTPA:
-
Diethylene-triamine-pentaacetate
- ELISpot:
-
Enzyme-linked immunospot
- 68Ga:
-
Gallium-68
- IFN:
-
Interferon
- IL:
-
Interleukin
- LTT:
-
Lymphocyte transformation test
- OKT3:
-
Anti-CD3 monoclonal antibody
- PBMCs:
-
Peripheral blood mononuclear cells
- PET/CT:
-
Positron emission tomography together with computed tomography
- 90Sr:
-
Strontium-90
- 90Y:
-
Yttrium-90
References
Perico ME, Chinol M, Nacca A, Luison E, Paganelli G, Canevari S (2001) The humoral immune response to macrocyclic chelating agent DOTA depends on the carrier molecule. J Nucl Med 42(11):1697–1703
Harrison A, Walker CA, Parker D et al (1991) The in vivo release of 90Y from cyclic and acyclic ligand-antibody conjugates. Int J Rad Appl Instrum B 18(5):469–476
Kozak RW, Raubitschek A, Mirzadeh S, Brechbiel MW, Junghans RP, Gansow OA, Waldmann TA (1989) Nature of the bifunctional chelating agent used for radioimmunotherapy with yttrium-90 monoclonal antibodies: critical factors in determining in vivo survival and organ toxicity. Cancer Res 49(10):2639–2644
Knox SJ, Goris ML, Trisler K et al (1996) Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin Cancer Res 2(3):457–470
Otte A, Mueller-Brand J, Dellas S, Nitzsche EU, Herrmann R, Maecke HR (1998) Yttrium-90-labelled somatostatin-analogue for cancer treatment. Lancet 351(9100):417–418
Rufini V, Calcagni ML, Baum RP (2006) Imaging of neuroendocrine tumors. Semin Nucl Med 36(3):228–247. doi:10.1053/j.semnuclmed.2006.03.007
Barsegian V, Müller SP, Horn PA, Bockisch A, Lindemann M (2011) Lymphocyte function following radioiodine therapy in patients with thyroid carcinoma. Nuklearmedizin 50(5):195–203. doi:10.3413/nukmed-04241108
Anderson RE, Warner NL (1976) Ionizing radiation and the immune response. Adv Immunol 24:215–335
Dehos G, Hinz G, Schwarz E-R (1986) Changes in number and function of the lymphocyte populations as a biological indicator for ionizing radiation. In: Kaul A, Dehos A, Bögl W, Hing G, Kossel F, Schwarz E-R, Stamm A, Stephan G (eds) Biological indicators for radiation dose assessment. MMV, München, pp 298–301
Guedeney G, Grunwald D, Malarbet JL, Doloy MT (1988) Time dependence of chromosomal aberrations induced in human and monkey lymphocytes by acute and fractionated exposure to 60Co. Radiat Res 116(2):254–262
Anderson RE, Lefkovits I (1980) Effects of irradiation on the in vitro immune response. Exp Cell Biol 48(4):255–278
Belka C, Ottinger H, Kreuzfelder E, Weinmann M, Lindemann M, Lepple-Wienhues A, Budach W, Grosse-Wilde H, Bamberg M (1999) Impact of localized radiotherapy on blood immune cells counts and function in humans. Radiother Oncol 50(2):199–204
Breitz H (2002) Dosimetry in a myeloablative setting. Cancer Biother Radiopharm 17(1):119–128. doi:10.1089/10849780252824136
Marincek N, Jorg AC, Brunner P et al (2013) Somatostatin-based radiotherapy with [90Y-DOTA]-TOC in neuroendocrine tumors: long-term outcome of a phase I dose escalation study. J Transl Med 11:17. doi:10.1186/1479-5876-11-17
IRCP (1984) Publication 51. Nonstochastic effects of ionizing radiation. Pergamon Press, Oxford
Coleman CN, Blakely WF, Fike JR et al (2003) Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiat Res 159(6):812–834
Russell CD, Bischoff PG, Kontzen FN, Rowell KL, Yester MV, Lloyd LK, Tauxe WN, Dubovsky EV (1985) Measurement of glomerular filtration rate: single injection plasma clearance method without urine collection. J Nucl Med 26(11):1243–1247
Lindemann M, Witzke O, Winterhagen T et al (2004) T-cell function after interleukin-2 therapy in HIV-infected patients is correlated with serum cortisol concentrations. AIDS 18(15):2001–2007
IRCP (1987) Publication 53 (with Addenda). Radiation dose to patients from radiopharmaceuticals. A report of a Task Group of Committee 2 of the International Commission on Radiological Protection. Ann ICRP 18 (1–4): 1–377
IRCP (1998) Publication 80: radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53). Ann ICRP 28(3):1–126
Förster GJ, Engelbach MJ, Brockmann JJ, Reber HJ, Buchholz HG, Macke HR, Rosch FR, Herzog HR, Bartenstein PR (2001) Preliminary data on biodistribution and dosimetry for therapy planning of somatostatin receptor positive tumours: comparison of (86)Y-DOTATOC and (111)In-DTPA-octreotide. Eur J Nucl Med 28(12):1743–1750. doi:10.1007/s002590100628
Hänscheid H, Lassmann M, Luster M et al (2006) Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med 47(4):648–654
Dale RG (1996) Dose-rate effects in targeted radiotherapy. Phys Med Biol 41(10):1871–1884
Vrisekoop N, den Braber I, de Boer AB et al (2008) Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc Natl Acad Sci USA 105(16):6115–6120. doi:10.1073/pnas.0709713105
Lindemann M, Belger P, Ottinger HD, Beelen DW, Grosse-Wilde H (2005) Long-term follow-up of cellular in vitro immunity after allogeneic peripheral blood stem cell versus bone marrow transplantation. Tissue Antigens 66(5):480 [Abstract]
Carr BI, Metes DM (2012) Peripheral blood lymphocyte depletion after hepatic arterial 90Yttrium microsphere therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 82(3):1179–1184. doi:10.1016/j.ijrobp.2010.10.042
Stevenson AF, Daculsi R, Monig H (1982) Haematological studies on 90Sr–90Y-toxicity: II. Femoral CFU-s kinetics and mitogen response of spleen cells. Radiat Environ Biophys 20(4):275–287
Birkeland SA (1976) The immunosuppressive effect of extracorporeal irradiation of the blood using a portable 90Sr–90Y source and small transit doses. Scand J Immunol 5(4):323–329
Lindemann M, Rebmann V, Ottinger HD, Schmolke K, Kreuzfelder E, Grosse-Wilde H (2004) rhG-CSF effect on mixed lymphocyte cultures and circulating soluble HLA antigen levels in volunteer stem cell donors. Exp Hematol 32(11):1103–1109. doi:10.1016/j.exphem.2004.07.025
Ugurel S, Lindemann M, Schadendorf D, Grosse-Wilde H (2004) Altered surface expression patterns of circulating monocytes in cancer patients: impaired capacity of T-cell stimulation? Cancer Immunol Immunother 53(11):1051
Acknowledgments
This article is a partial fulfillment of requirements for the doctor’s degree at the Medical Faculty, University of Essen, Germany, for Mr. C. Hueben.
Ethical standards
All patients provided written informed consent prior to their inclusion in the study. The study was approved by the institutional review board and carried out in accordance with the Helsinki Declaration of 1964, as revised in 2000.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barsegian, V., Hueben, C., Mueller, S.P. et al. Impairment of lymphocyte function following yttrium-90 DOTATOC therapy. Cancer Immunol Immunother 64, 755–764 (2015). https://doi.org/10.1007/s00262-015-1687-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1687-3