Abstract
Introduction
There is mounting evidence describing the immunosuppressive role of bulky metastatic disease, thus countering the therapeutic effects of tumor vaccine. Therefore, adjuvant immunotherapy may have a better impact on clinical outcome. In this phase II clinical trial, we aimed to test the feasibility of using a specific mutant ras peptide vaccine as an adjuvant immunotherapy in pancreatic and colorectal cancer patients.
Materials and methods
Twelve patients with no evidence of disease (NED), five pancreatic and seven colorectal cancer patients were vaccinated subcutaneously with 13-mer mutant ras peptide, corresponding to their tumor’s ras mutation. Vaccinations were given every 4 weeks, up to a total of six vaccines.
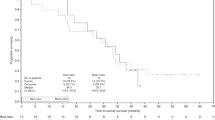
Results
No serious acute or delayed systemic side effects were seen. We detected specific immune responses to the relevant mutant ras peptide by measuring IFN-gamma mRNA expression by quantitative real-time PCR. Five out of eleven patients showed a positive immune response. Furthermore, the five pancreatic cancer patients have shown a mean disease-free survival (DFS) of 35.2+ months and a mean overall survival (OS) of 44.4+ months. The seven colorectal cancer patients have shown a mean disease-free survival (DFS) of 27.2+ months and a mean overall survival (OS) of 41.5+ months.
Conclusion
In this study, we found that it is feasible to use mutant ras vaccine in the adjuvant setting. This vaccine is safe, can induce specific immune responses, and it appears to have a positive outcome in overall survival. Therefore, we believe that such an approach warrants further investigation in combination with other therapies.



Similar content being viewed by others
References
Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN et al (1993) K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol 143(2):545–554
Li ZH, Zheng J, Weiss LM, Shibata D (1994) c-k-ras and p53 mutations occur very early in adenocarcinoma of the lung. Am J Pathol 144(2):303–309
Breivik J, Meling GI, Spurkland A, Rognum TO, Gaudernack G (1994) K-ras mutation in colorectal cancer: relations to patient age, sex and tumour location. Br J Cancer 69(2):367–371
Weiss S, Bogen B (1991) MHC class II-restricted presentation of intracellular antigen. Cell 64(4):767–776
Jardetzky TS, Lane WS, Robinson RA, Madden DR, Wiley DC (1991) Identification of self peptides bound to purified HLA-B27. Nature 353(6342):326–329
Abrams SI, Stanziale SF, Lunin SD, Zaremba S, Schlom J (1996) Identification of overlapping epitopes in mutant ras oncogene peptides that activate CD4+ and CD8+ T cell responses. Eur J Immunol 26(2):435–443
Fossum B, Gedde-Dahl T 3rd, Breivik J, Eriksen JA, Spurkland A, Thorsby E et al (1994) p21-ras-peptide-specific T-cell responses in a patient with colorectal cancer. CD4+ and CD8+ T cells recognize a peptide corresponding to a common mutation (13Gly → Asp). Int J Cancer 56(1):40–45
Fossum B, Olsen AC, Thorsby E, Gaudernack G (1995) CD8+ T cells from a patient with colon carcinoma, specific for a mutant p21-Ras-derived peptide (Gly13 → Asp), are cytotoxic towards a carcinoma cell line harbouring the same mutation. Cancer Immunol Immunother 40(3):165–172
Gedde-Dahl T 3rd, Fossum B, Eriksen JA, Thorsby E, Gaudernack G (1993) T cell clones specific for p21 ras-derived peptides: characterization of their fine specificity and HLA restriction. Eur J Immunol 23(3):754–760
Van Elsas A, Nijman HW, Van der Minne CE, Mourer JS, Kast WM, Melief CJ et al (1995) Induction and characterization of cytotoxic T-lymphocytes recognizing a mutated p21ras peptide presented by HLA-A*0201. Int J Cancer 61(3):389–396
Abrams SI, Khleif SN, Bergmann-Leitner ES, Kantor JA, Chung Y, Hamilton JM et al (1997) Generation of stable CD4+ and CD8+ T cell lines from patients immunized with ras oncogene-derived peptides reflecting codon 12 mutations. Cell Immunol 182(2):137–151
Khleif SN, Abrams SI, Hamilton JM, Bergmann-Leitner E, Chen A, Bastian A et al (1999) A phase I vaccine trial with peptides reflecting ras oncogene mutations of solid tumors. J Immunother 22(2):155–165
Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Solheim BG, Soreide O et al (1995) Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet 346(8987):1399–1400
Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Soreide O et al (2001) Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer 92(3):441–450
Carbone DP, Ciernik IF, Kelley MJ, Smith MC, Nadaf S, Kavanaugh D et al (2005) Immunization with mutant p53- and K-ras-derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol 23:5099–5107
Hsieh CL, Chen DS, Hwang LH (2000) Tumor-induced immunosuppression: a barrier to immunotherapy of large tumors by cytokine-secreting tumor vaccine. Hum Gene Ther 11(5):681–692
Mocellin S, Wang E, Marincola FM (2001) Cytokines and immune response in the tumor microenvironment. J Immunother 24(5):392–407
Mocellin S, Rossi CR, Lise M, Marincola FM (2002) Adjuvant immunotherapy for solid tumors: from promise to clinical application. Cancer Immunol Immunother 51(11–12):583–595
Hoover HC Jr, Brandhorst JS, Peters LC, Surdyke MG, Takeshita Y, Madariaga J et al (1993) Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J Clin Oncol 11(3):390–399
Harris JE, Ryan L, Hoover HC Jr, Stuart RK, Oken MM, Benson AB 3rd et al (2000) Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern cooperative oncology group study E5283. J Clin Oncol 18(1):148–157
Kammula US, Lee KH, Riker AI, Wang E, Ohnmacht GA, Rosenberg SA et al (1999) Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol 163(12):6867–6875
Rothman KJ (1978) Estimation of confidence limits for the cumulative probability of survival in life table analysis. J Chronic Dis 31(8):557–560
Provenzano M, Mocellin S, Bettinotti M, Preuss J, Monsurro V, Marincola FM et al (2002) Identification of immune dominant cytomegalovirus epitopes using quantitative real-time polymerase chain reactions to measure interferon-gamma production by peptide-stimulated peripheral blood mononuclear cells. J Immunother 25(4):342–351
Provenzano M, Mocellin S, Bonginelli P, Nagorsen D, Kwon SW, Stroncek D (2003) Ex vivo screening for immunodominant viral epitopes by quantitative real time polymerase chain reaction (qRT-PCR). J Transl Med 1(1):12
Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR et al (2001) Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 19(1):145–156
Acknowledgments
The investigators thank Dr. Jay A. Berzofsky, Dr. Scott Abrams and Dr. Walter Urba for the valuable review of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toubaji, A., Achtar, M., Provenzano, M. et al. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers . Cancer Immunol Immunother 57, 1413–1420 (2008). https://doi.org/10.1007/s00262-008-0477-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0477-6