Abstract
Purpose
Since its introduction in 2011, FDG-PET/MRI has been advocated as a useful adjunct in colorectal cancer care. However, gaps and limitations in current research remain. This systematic review aims to review the current literature to quantify the utility of FDG-PET/MRI in colorectal cancer care.
Methods
An up-to-date review was performed on the available literature between 2000 and 2023 on PubMed, EMBASE, Medline, databases. All studies reporting on the use of FDG-PET/MRI in colorectal cancer care were analyzed. The main outcome measures were accuracy in initial staging, restaging, and detection of metastatic disease in both rectal as well as colon cancers. The secondary outcome was comparing the performance of FDG-PET/MRI versus Standard of Care Imaging (SCI). Finally, the clinical significance of FDG-PET/MRI was measured in the change in management resulting from imaging findings.
Results
A total of 22 observational studies were included, accounting for 988 patients. When individually compared to current Standard of Care Imaging (SCI)—MRI pelvis for rectal cancer and thoraco-abdominal contrast CT, PET/MRI proved superior in terms of distant metastatic disease detection. This led to as much as 21.0% change in management. However, the technological limitations of PET/MRI were once again highlighted, suggesting SCI should retain its place as first-line imaging.
Conclusion
FDG-PET/MRI appears to be a promising adjunct in staging and restaging of colorectal cancer in carefully selected patients.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the management of colorectal cancer, it is vital to obtain an accurate Tumor, Node and Metastasis (TNM) staging to allow for an individualized treatment approach [1, 2]. This aids in complex discussion particularly in locally advanced rectal cancer (LARC) for which clear oncological margins (distal and circumferential resection margin) is paramount. The routine pre-operative work-up (Standard of Care Imaging) for newly diagnosed colorectal cancer patients include computed tomography (CT) to ensure no distant metastasis, magnetic resonance imaging (MRI) for those with mid to low rectal cancers and additionally [18F] Fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT if there is concern for any occult residual disease that has not been identified by the first two modalities.
Currently, the treatment algorithm for mid to low rectal cancer is evolving [3]. Historically, any patients with LARC, defined as T3-4 with or without nodal positivity, will either receive neoadjuvant short-course radiotherapy or long-course chemoradiotherapy. However, in the last 2 years, there has been increasing interest in Total Neoadjuvant Therapy (TNT): giving induction (before radiotherapy) or consolidation (after radiotherapy) chemotherapy before definitive curative radical surgery [4, 5]. The basis for this intensified regimen is to bring forward neoadjuvant chemotherapy as patients have higher functional reserves and would better tolerate chemotherapy before major surgery. This would hypothetically reduce the risk of distant recurrence.
Recent intermediate oncological data has shown improvement in 3-year disease-free survival, with an absolute reduction of approximately 7% when chemotherapy was given before surgery compared to after surgery [5, 6]. Most importantly, patients had less chemotherapeutic toxicity and consequently increased compliance rates. Furthermore, there is also interest in organ preservation after neoadjuvant therapy in those with clinically complete response, defined as no evidence of rectal cancer on digital rectal examination, endoscopic assessment, and MRI and/or FDG-PET/CT [7].
This has reignited interest in the use of [18F] Fluorodeoxyglucose- positron emission tomography /MRI (FDG-PET/MRI) as an effective imaging modality to improve accuracy of staging in colorectal cancer. It was first approved for clinical use back in 2011 in the United States of America and sought to combine the strengths of both PET and MRI modalities. It harnesses the strengths of MRI in defining anatomy, and offers diffusion- weighted imaging (DWI), spectroscopy, blood oxygenation level-dependent imaging in functional MRI, T1/T2 mapping and dynamic contrast-enhanced imaging. Contrast-enhanced MRI is an established imaging modality that has numerous clinical applications due to its superb soft tissue contrast and lack of ionizing radiation, and the ability to assess cellular density by diffusion-weighted imaging (DWI) and tissue perfusion by dynamic contrast-enhanced (DCE) [6, 8, 9]. Importantly, this has the potential to complement the metabolic imaging provided by FDG-PET. This assessment of metabolic activity supplements size/ morphology information and developed protocols have shortened scanning time and allowed correction of movement, hence improving the appeal of this imaging modality as an adjunct to colorectal cancer care [10].
Certainly, different protocols have been developed to streamline the acquisition process of PET/MRI—to save patient/scanner time while optimizing the PET/MRI sequence. The focus has been on timing MRI sequence to better match each PET bed position without compromising diagnostic quality so the images are acquired simultaneously. This process commences with a localizer, comparable to the topogram in a PET/CT scan, to be used for planning both the PET bed positions and the MRI sequences at each bed position. Thereafter, a specific MRI attenuation correction sequence is obtained at each bed position to create the attenuation correction maps for PET reconstruction. Finally, depending on the region being imaged and the pathology of interest, several other sequences have been developed to be performed at each bed position. This allows the complementary advantages of FDG-PET and MRI to theoretically improve our diagnostic accuracy and treatment decision-making for colorectal cancer [11].
It is also worth noting that FDG-PET/MRI does involve radiation exposure compared to MRI and PET/MRI has a significantly lower radiation dose compared to PET/CT, which is particularly beneficial in younger patients [12]. Therefore, the combination of FDG-PET and MRI in an integrated (hybrid) FDG-PET/MRI system promises to have a positive impact on disease diagnosis, staging, and restaging [13].
Its high sensitivity and its efficacy have been reported in the accurate localization of various urological and gynecological cancers [14, 15] but a wide consensus on the clinical usefulness of FDG-PET/MRI in the staging and restaging of colorectal cancer patients has yet to be reached. This systematic review aims to evaluate the effectiveness, summarizing the existing literature and discuss the emerging role of hybrid FDG-PET/MRI in colorectal malignancies which is currently underutilized.
Methods
Search strategy
A computer-assisted search of four databases (PubMed, EMBASE, Medline) were conducted for articles published between the years of 2000 and 2023. The following medical subject heading (MeSH) terms and text words were used for the search in all possible combinations: “PET/MRI” AND “colorectal cancer” OR “colorectal cancer imaging”. The cited references in each retrieved paper were also checked to ensure that all publications relevant to this study were captured. The last search date for this study was 30 June 2023.
Selection of studies
This study was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [16]. Two reviewers (H.L. and K.C.) screened all article titles and abstracts, with all potentially relevant studies then subsequently selected for full text review. The selection of articles was based on the following inclusion criteria: adult population, all prospective or retrospective studies reporting on the performance of FDG-PET/MRI—accuracy in initial staging, restaging, and detection of metastatic disease, in addition to comparison with Standard of Care imaging (SCI) in colorectal cancer care. Studies that did not report on the performance of FDG-PET/MRI and SCI were excluded. All non-English studies, letters and conference abstracts were also excluded.
Data extraction
Two reviewers independently extracted the data from the included studies using a standard data extraction form based on the PRISMA guidelines. Extracted information included those related to study information: country, study type, time period; study population and participant demographics: sample size, age, gender, imaging modalities utilized; indication of imaging; sensitivity and specificity of imaging modalities utilized; positive and negative predictive values of imaging modalities utilized and changes in management. Any discrepancies were resolved by consensus between the two reviewers and the supervising author (J.K.).
Results
Study characteristics
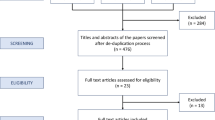
Our search identified 85 non-duplicate and relevant studies from electronic databases. After screening titles and abstracts, 31 were identified for full-text reading and 22 of these articles were eventually included in our study cohort (Fig. 1) [11, 13, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
Methodological quality
All 23 studies were assessed with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool using four domains—patient selection, index test, reference standard and flow and timing. The risk of bias for patient selection was high in many papers. Correspondingly, the flow and timing were also high.
Clinical research design
There were ten prospective studies, eleven retrospective studies and one pilot study (Table 1). This accounted for a total of 988 patients, with 582 rectal cancer patients. Disease staging was the most common indication for the scan, either on initial diagnosis or after neoadjuvant Chemoradiotherapy (nCRT) in rectal cancer.
Indeed in 22 studies, the main endpoint was the ability of FDG-PET/MRI to stage colorectal cancer, be it primary or local recurrent disease. A comparison was drawn between FDG-PET/CT and FDG-PET/MRI performed in the same populations in ten studies. A comparison was drawn between FDG-PET/MRI and MRI performed in the same populations in seven studies.
PET/MRI for initial staging
In this present review, 22 studies assessed FDG-PET/MRI use in the staging setting for the purpose of detecting burden of primary disease.
Primary tumor
Integrated FDG-PET/MRI proved to be of greater diagnostic value. While the high soft tissue resolution provided by MRI allows for reliable assessment of T stage and the circumferential resection margin, the combination of FDG-PET with MRI can help in both lesion detection as well as margin delineation of the primary tumor planning beyond the muscularis propria [17, 18, 25].
Nodal disease
Compared to MRI alone, FDG-PET/MRI N-staging accuracy was once again superior: 78.3% to 79% for FDG-PET/MRI compared to 58% to 76.1% for MRI [17, 23]. This represents the greatest advantage of FDG-PET/MRI as one of the central issues in current rectal cancer management is the accurate characterization of small lymph nodes.
Colorectal cancer
There were 8 studies analyzing the accuracy of staging for colorectal cancer [13, 20, 25, 29, 30, 32, 34, 36] (Table 2). Overall, the detection of metastatic disease by FDG-PET/MRI was higher at 69% to 99.5% compared to 47.5% to 71% for other imaging techniques like FDG-PET/CT, CT or MRI alone [13, 20, 29].
Rectal cancer
There were 12 studies analyzing the accuracy of staging for rectal cancer (Table 3) [11, 17,18,19, 22,23,24, 26,27,28, 33, 35]. Overall, FDG-PET/MRI accuracy was superior at 88.4% to 93% compared to 82.6% to 85% for conventional imaging modalities MRI and CT. When comparing FDG-PET/MRI with CT for Tumor (T) and Node (N) staging, FDG-PET/MRI had higher accuracy in both aspects (92%, 92% vs 89%, 86%).
PET/MRI vs PET/CT
FDG-PET/MRI and FDG-PET/CT were directly compared in 10 papers, all focused on systemic staging in the detection of distant metastasis (Table 4) [13, 18, 20, 25, 28, 29, 33,34,35,36]. The sensitivity of FDG-PET/MRI was higher than that of FDG-PET/CT, ranging from 59 to 94% while FDG-PET/CT ranges from 58 to 88%. The specificity of FDG-PET/MRI ranges from 87 to 100% while FDG-PET/CT ranges from 75 to 100% [18, 29, 33].
PET/MRI vs MRI
FDG-PET/MRI and MRI were compared in 7 papers (Table 5) [11, 13, 17, 18, 23, 29, 35]. Three utilized FDG-PET/MRI to assess T and N stage while the remaining four assessed their performance in detecting metastatic disease. Combined, the sensitivity of combined FDG-PET/MRI was comparable to that of MRI, ranging from 59% to 90.8% versus 58% to 98.5% respectively with slightly superior specificity: 86.1% to 87% compared to 66.7% to 94% [11, 18, 29]. The addition of metabolic imaging in FDG-PET/MRI proved superior in detecting distant metastatic disease with significantly higher sensitivity for M staging (100% vs 93.8%). While showing slightly lower sensitivity for N staging (72.7% vs 68.2%), FDG-PET/MRI provided higher specificity for N staging (87.5% vs 79.2%) while MRI provided higher specificity for M staging (89.2% vs 84.8%).
Change in management from using PET/MRI
Importantly, this translated to changes in management after FDG-PET/MRI identified disease not picked up previously. Nine papers reported changes in management that ranged from 7.1% to 21.6%, with a majority reporting upstaging which resulted in further surgeries or abortion of surgeries for chemotherapy instead [11, 13, 20, 22, 25, 28, 30, 34, 36]. This was based on pathological confirmation of nodal and distant disease and while the overall rates of false positives or false negatives were not reported, this deserves greater attention.
Discussion
Our updated systematic review on FDG-PET/MRI use in colorectal cancer care has highlighted the potential for it to change management in up to 7.1% to 21.6% of cases. This significant advantage of FDG-PET/MRI with regards to lesion detection and characterization (T stage) as well as in quantifying N and M staging suggest that FDG-PET/MRI is an emerging modality that may prove useful in the management of colorectal cancer patients, supporting the move towards hybrid imaging modalities which have proven to be advantageous in other cancers.
Specifically, this change in management can be attributed to the higher soft tissue contrast from FDG-PET/MRI which confers higher sensitivity and accuracy in detecting involved lymph nodes. N staging requires more than anatomic imaging as metastatic lymph nodes can have similar appearance and size as a normal non-metastatic lymph node. Hence, FDG-PET is imperative for such diagnostic purposes because the standardized uptake values (SUV) of the metastatic lymph nodes are higher than normal lymph nodes [9, 37]. At the same time, combining FDG-PET with MRI can help better characterize small pelvic nodes < 1 cm, overcoming the limitation of PET/CT in borderline enlarged lymph nodes between 5 and 10 mm [38, 39]. In particular, the longer FDG-PET acquisition that is simultaneous to the MRI sequences of the pelvis appears to result in a higher sensitivity for small perirectal nodes, particularly those that measure less than 5 mm [40]. Together, this has contributed to the significant percentages of change in management as shown in our review. The majority of patients with change in management had been reported as upstaging, resulting in further surgeries or abortion of surgeries for chemotherapy instead. This shows the potential of FDG-PET/MRI in detecting advanced diseases that is not surgically indicated, allowing patients to avoid unnecessary surgical interventions.
In the assessment of the M stage, our study determined a clear superiority of FDG PET/MRI in comparison to CT both in the staging and detection of metastatic disease. Currently, MRI has established itself as the reference imaging technique for metastasis, particularly in colorectal liver metastases [41]. With PET/MRI, it further allows for increased accuracy in detecting hepatic masses in colorectal cancer by combining the multiphase MRI of the liver together with PET which can potentially provide increased sensitivity and specificity [20, 21]. This is particularly useful when assessing liver lesions that would have otherwise been too small to characterize or remained indeterminate on CT [41, 42]. To mitigate the known intrinsic weakness of MRI in identifying the metastatic lung lesions, new, specific sequences have recently been developed to address this issue. The use of ultra-short echo times (UTE) sequences has provided superior visualization of lung parenchyma, achieving greater sensitivity in the detection of lung lesions [43, 44]. In a study done in 2022, UTE lung MRI showed close to 100% detection rate of nodules that were 5 mm or more in size but lower detection rate for nodules that were less than 5 mm in size. High resolution CT would then be more preferable in detecting metastasis in cancer patients but transition to UTE lung MRI should be done when following-up due to ionizing radiation exposure [45].
With the emerging paradigm shift in rectal cancer treatment with total neoadjuvant therapy (TNT) and watch/wait strategies proving non-inferiority in patients with clinical complete response (cCR), the accurate selection of patients with cCR has never been more crucial. Historically, FDG-PET/CT has shown sensitivity and specificity of 71% and 76%, respectively in predicting a histopathologically complete response after nCRT for rectal cancer [46]. It has been suggested that FDG-PET/MRI, by combining the already-reported good accuracy of PET images with the anatomical detail of MRI, could potentially improve on that [9, 38].
At present, the partial or complete replacement of viable tumor tissue with fibrosis has led to under-staging in some cases due to the inability of FDG-PET/CT to detect small clusters of residual disease or failure of morphological sequences (such as conventional T2-weighted MRI) and functional ones (such as diffusion-weighted imaging) to identify small remnant tumor deposits in the initial tumor site. By combining morphological, functional, and metabolic data, FDG-PET/MRI would be able to overcome these shortfalls and more accurately re-stage patients after nCRT [46, 47]. The integration of radiomics in an artificial intelligent model promises to enhance this capability. Recently, Giannini et al. compared 52 patients with locally advanced rectal cancer and how their histology results corresponded to texture features from FDG-PET/CT and MRI images [48]. Building on this, other studies have combined FDG-PET and MRI radiomics features together and showed greater sensitivity/specificity over models including only MRI features [42].
Equally, the authors acknowledge the inherent limitations of FDG-PET/MRI technology. While the main drawback of long acquisition time averaging 30 min, as well as the high costs have been widely reported, there are still several technical considerations required in the design and operation of the imaging systems. Specifically, modifications to conventional imaging systems that accommodate integration of the two modalities without image-degrading cross talk require deeper understanding before the technology can be widely adopted. Furthermore, there would be technical difficulties involved as PET detectors and not compatible with magnetic fields that will be present in MRI [49]. The integration of FDG-PET/MRI affords the opportunity for enhanced protocols and clinical applications which are currently being explored to overcome this [9].
In FDG-PET imaging, the limitations of standardized uptake value (SUV) cutoff, commonly used as a semi-quantitative value that measures tissue radioactivity concentration hence determining positive disease, could be overcome by volume-based FDG-PET/MRI parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) to measure metabolic activity in the entire tumor mass. Our review confirmed the widely accepted SUV cutoff between benign and malignant lesions with a range from 2.0 to 2.5. While percentage change in tumor SUVmax and changes in DWI post-neoadjuvant therapy are predictors of outcome, it may not reflect the heterogeneous nature of the tumor. MTV represents the dual characteristics of tumor volume and extent of FDG uptake by tumor tissues. TLG has been proposed as a more accurate parameter as it considers SUVmean and MTV [50]. With the smaller slices during longer PET acquisition phase in PET/MRI, it is hoped this will decrease the partial volume artifact with future studies eagerly awaited. These changes in metabolism seen on FDG-PET could complement the information gathered on MRI alone.
Our analysis also showed that the specificity of FDG-PET/MRI is lower than MRI in the detection of metastatic disease. However, FDG-PET/MRI has a large potential in this field considering its ability to pick up metastatic disease that was previously not picked up with SCI, leading to fewer unnecessary surgeries. Future larger studies should research deeper on the use of FDG-PET/MRI and its potential for management changes.
Finally, we acknowledge the scarce number of studies published on this topic and the low level of evidence for most of the included studies. These studies have often included colon and rectal cancer patients together. However, the important suggestions of its role in changing management call for a Randomized Control Trial (RCT)—with FDG/PET-MRI in one arm and standard care imaging in the other to determine the overall impact on patient outcome in terms of survival benefit for example.
Conclusion
This combination of superior soft-tissue resolution of MRI with the picomolar sensitivity of FDG-PET brings great promise. Our findings support the importance of FDG-PET/MRI in the diagnosis and management of colorectal cancer, where FDG-PET/MR altered clinical management in up to 21% of cases. Further RCTs should consider the above in evaluating long term benefits/costs of this modality while developing protocols for its standardized use.
As we march towards an era of precision medicine in which the profiling of tumor types guides progressively more specifically targeted cytotoxic and biologic agents, it has driven demand for more accurate imaging modalities. While Artificial Intelligence (AI), radiomics and radio-genomics might present helpful adjuncts, the introduction of FDG-PET/MRI to the current clinical management of colorectal malignancies presents another pathway for a patient-centered treatment approach [13].
Data availability
The data that support the findings of this study are available on request from the corresponding author, H.L. The list of included articles have been included in the reference list.
References
Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW, et al. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Annals of surgical oncology. 2015;22:3873-80.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA: a cancer journal for clinicians. 2017;67(2):93-9.
Kong JC, Soucisse M, Michael M, Tie J, Ngan SY, Leong T, et al. Total neoadjuvant therapy in locally advanced rectal cancer: a systematic review and metaanalysis of oncological and operative outcomes. Annals of Surgical Oncology. 2021;28(12):7476-86.
Hospers G, Bahadoer RR, Dijkstra EA, van Etten B, Marijnen C, Putter H, et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. American Society of Clinical Oncology; 2020.
Conroy T, Bosset J-F, Etienne P-L, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology. 2021;22(5):702-15.
Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CA, Putter H, Kranenbarg EM-K, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. The Lancet Oncology. 2021;22(1):29-42.
Habr-Gama A, Gama-Rodrigues J, São Julião GP, Proscurshim I, Sabbagh C, Lynn PB, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. International Journal of Radiation Oncology* Biology* Physics. 2014;88(4):822–8.
Zhang C, Liang Z, Liu W, Zeng X, Mo Y. Comparison of whole-body 18F-FDG PET/CT and PET/MRI for distant metastases in patients with malignant tumors: a meta-analysis. BMC cancer. 2023;23(1):1-14.
Crimi F, Valeggia S, Baffoni L, Stramare R, Lacognata C, Spolverato G, et al. [18F] FDG PET/MRI in rectal cancer. Annals of Nuclear Medicine. 2021;35:281-90.
Nensa F, Beiderwellen K, Heusch P, Wetter A. Clinical applications of PET/MRI: current status and future perspectives. Diagn Interv Radiol. 2014;20(5):438-47.
Queiroz MA, Ortega CD, Ferreira FR, Nahas SC, Cerri GG, Buchpiguel CA. Diagnostic accuracy of FDG-PET/MRI versus pelvic MRI and thoracic and abdominal CT for detecting synchronous distant metastases in rectal cancer patients. European journal of nuclear medicine and molecular imaging. 2021;48:186-95.
Grueneisen J, Sawicki LM, Schaarschmidt BM, Suntharalingam S, von der Ropp S, Wetter A, et al. Evaluation of a Fast Protocol for Staging Lymphoma Patients with Integrated PET/MRI. PLOS ONE. 2016;11(6):e0157880.
Furtado FS, Suarez-Weiss KE, Vangel M, Clark JW, Cusack JC, Hong T, et al. Clinical impact of PET/MRI in oligometastatic colorectal cancer. British Journal of Cancer. 2021;125(7):975-82.
Partovi S, Kohan A, Rubbert C, Vercher-Conejero JL, Gaeta C, Yuh R, et al. Clinical oncologic applications of PET/MRI: a new horizon. American journal of nuclear medicine and molecular imaging. 2014;4(2):202.
Bashir U, Mallia A, Stirling J, Joemon J, MacKewn J, Charles-Edwards G, et al. PET/MRI in oncological imaging: state of the art. Diagnostics. 2015;5(3):333-57.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097.
Catalano OA, Lee SI, Parente C, Cauley C, Furtado FS, Striar R, et al. Improving staging of rectal cancer in the pelvis: the role of PET/MRI. European Journal of Nuclear Medicine and Molecular Imaging. 2021;48:1235-45.
Plodeck V, Platzek I, Streitzig J, Nebelung H, Blum S, Kühn J-P, et al. Diagnostic performance of 18F-fluorodeoxyglucose-PET/MRI versus MRI alone in the diagnosis of pelvic recurrence of rectal cancer. Abdominal Radiology. 2021;46(11):5086-94.
Queiroz MA, Ortega CD, Ferreira FR, Capareli FC, Nahas SC, Cerri GG, et al. Value of primary rectal tumor PET/MRI in the prediction of synchronic metastatic disease. Molecular imaging and biology. 2021:1–11.
Zhou N, Guo X, Sun H, Yu B, Zhu H, Li N, et al. The value of 18F-fdg PET/CT and abdominal PET/MRI as a one-stop protocol in patients with potentially resectable colorectal liver metastases. Frontiers in oncology. 2021;11:714948.
Zhang Y-N, Lu X, Lu Z-G, Fu L-P, Zhao J, Xiang Z-L. Evaluation of hybrid PET/MRI for gross tumor volume (GTV) delineation in colorectal cancer liver metastases radiotherapy. Cancer Management and Research. 2021:5383–9.
Crimì F, Spolverato G, Lacognata C, Garieri M, Cecchin D, Urso ED, et al. 18F-FDG PET/MRI for rectal cancer TNM restaging after preoperative chemoradiotherapy: initial experience. Diseases of the Colon & Rectum. 2020;63(3):310-8.
Li Y, Mueller LI, Neuhaus JP, Bertram S, Schaarschmidt BM, Demircioglu A, et al. 18F-FDG PET/MR versus MR alone in whole-body primary staging and restaging of patients with rectal cancer: what is the benefit of PET? Journal of Clinical Medicine. 2020;9(10):3163.
Yoon JH, Lee JM, Chang W, Kang H-j, Bandos A, Lim H-j, et al. Initial M staging of rectal cancer: FDG PET/MRI with a hepatocyte-specific contrast agent versus contrast-enhanced CT. Radiology. 2020;294(2):310-9.
Amorim BJ, Hong TS, Blaszkowsky LS, Ferrone CR, Berger DL, Bordeianou LG, et al. Clinical impact of PET/MR in treated colorectal cancer patients. European journal of nuclear medicine and molecular imaging. 2019;46:2260-9.
Ferri V, Vicente Lopez E, Quijano Collazo Y, Caruso R, Duran Gimenez Rico H, Ielpo B, et al. Quantitative analysis of 18-FDG-PET/MRI to assess pathological complete response following neoadjuvant radiochemotherapy in locally advanced rectal cancer. A prospective preliminary study. Acta Oncologica. 2019;58(9):1246-9.
Plodeck V, Rahbari NN, Weitz J, Radosa CG, Laniado M, Hoffmann R-T, et al. FDG-PET/MRI in patients with pelvic recurrence of rectal cancer: first clinical experiences. European Radiology. 2019;29:422-8.
Rutegård MK, Båtsman M, Axelsson J, Brynolfsson P, Brännström F, Rutegård J, et al. PET/MRI and PET/CT hybrid imaging of rectal cancer–description and initial observations from the RECTOPET (REctal Cancer trial on PET/MRI/CT) study. Cancer Imaging. 2019;19:1-9.
Brendle C, Schwenzer N, Rempp H, Schmidt H, Pfannenberg C, La Fougère C, et al. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. European journal of nuclear medicine and molecular imaging. 2016;43:123-32.
Kang B, Lee JM, Song YS, Woo S, Hur BY, Jeon JH, et al. Added value of integrated whole-body PET/MRI for evaluation of colorectal cancer: comparison with contrast-enhanced MDCT. American Journal of Roentgenology. 2016;206(1):W10-W20.
Lee DH, Lee JM, Hur BY, Joo I, Yi N-J, Suh K-S, et al. Colorectal cancer liver metastases: diagnostic performance and prognostic value of PET/MR imaging. Radiology. 2016;280(3):782-92.
Lee SJ, Seo HJ, Kang KW, Jeong S-Y, Yi N-J, Lee JM, et al. Clinical performance of whole-body 18F-FDG PET/Dixon-VIBE, T1-weighted, and T2-weighted MRI protocol in colorectal cancer. Clinical Nuclear Medicine. 2015;40(8):e392-e8.
Paspulati RM, Partovi S, Herrmann KA, Krishnamurthi S, Delaney CP, Nguyen NC. Comparison of hybrid FDG PET/MRI compared with PET/CT in colorectal cancer staging and restaging: a pilot study. Abdominal imaging. 2015;40:1415-25.
Al-Nabhani KZ, Syed R, Michopoulou S, Alkalbani J, Afaq A, Panagiotidis E, et al. Qualitative and quantitative comparison of PET/CT and PET/MR imaging in clinical practice. Journal of Nuclear Medicine. 2014;55(1):88-94.
Kam M, Wong D, Siu S, Stevenson A, Lai J, Phillips G. Comparison of magnetic resonance imaging–fluorodeoxy-glucose positron emission tomography fusion with pathological staging in rectal cancer. Journal of British Surgery. 2010;97(2):266-8.
Catalano OA, Coutinho AM, Sahani DV, Vangel MG, Gee MS, Hahn PF, et al. Colorectal cancer staging: comparison of whole-body PET/CT and PET/MR. Abdominal Radiology. 2017;42:1141-51.
Langman G, Patel A, Bowley DM. Size and distribution of lymph nodes in rectal cancer resection specimens. Diseases of the Colon & Rectum. 2015;58(4):406-14.
Hope TA, Kassam Z, Loening A, McNamara MM, Paspulati R. The use of PET/MRI for imaging rectal cancer. Abdominal Radiology. 2019;44:3559-68.
Dresen RC, Beets GL, Rutten HJ, Engelen SM, Lahaye MJ, Vliegen RF, et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy part I. Are we able to predict tumor confined to the rectal wall? Radiology. 2009;252(1):71–80.
Bailey JJ, Jordan EJ, Burke C, Ohliger MA, Wang ZJ, Loon KV, et al. Does extended PET acquisition in PET/MRI rectal cancer staging improve results? American Journal of Roentgenology. 2018;211(4):896-900.
Karaosmanoglu AD, Onur MR, Ozmen MN, Akata D, Karcaaltincaba M, editors. Magnetic resonance imaging of liver metastasis. Seminars in Ultrasound, CT and MRI; 2016: Elsevier.
Capelli G, Campi C, Bao QR, Morra F, Lacognata C, Zucchetta P, et al. 18F-FDG-PET/MRI texture analysis in rectal cancer after neoadjuvant chemoradiotherapy. Nuclear Medicine Communications. 2022;43(7):815.
Crimì F, Varotto A, Orsatti G, Lacognata C, Cecchin D, Frigo A, et al. Lung visualisation on PET/MRI: implementing a protocol with a short echo-time and low flip-angle volumetric interpolated breath-hold examination sequence. Clinical Radiology. 2020;75(3):239. e15-. e21.
Burris NS, Johnson KM, Larson PE, Hope MD, Nagle SK, Behr SC, et al. Detection of small pulmonary nodules with ultrashort echo time sequences in oncology patients by using a PET/MR system. Radiology. 2016;278(1):239-46.
Periaswamy G, Arunachalam VK, Varatharajaperumal R, Kalyan G, Selvaraj R, Mehta P, et al. Comparison of Ultrashort TE Lung MRI and HRCT Lungs for Detection of Pulmonary Nodules in Oncology Patients. Indian J Radiol Imaging. 2022;32(4):497-504.
Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D. Value of 18F-FDG PET for predicting response to neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. American Journal of Roentgenology. 2015;204(6):1261-8.
Gassert FG, Rübenthaler J, Cyran CC, Rink JS, Schwarze V, Luitjens J, et al. 18 F FDG PET/MRI with hepatocyte-specific contrast agent for M staging of rectal cancer: a primary economic evaluation. European Journal of Nuclear Medicine and Molecular Imaging. 2021;48:3268-76.
Giannini V, Mazzetti S, Bertotto I, Chiarenza C, Cauda S, Delmastro E, et al. Predicting locally advanced rectal cancer response to neoadjuvant therapy with 18 F-FDG PET and MRI radiomics features. European journal of nuclear medicine and molecular imaging. 2019;46:878-88.
Queiroz MA, Barbosa FdG, Buchpiguel CA, Cerri GG. Positron emission tomography/magnetic resonance imaging (PET/MRI): An update and initial experience at HC-FMUSP. Revista da associacao medica brasileira. 2018;64(1):71-84.
Avallone A, Aloj L, Pecori B, Caracò C, De Stefano A, Tatangelo F, et al. 18F-FDG PET/CT is an early predictor of pathologic tumor response and survival after preoperative radiochemotherapy with bevacizumab in high-risk locally advanced rectal cancer. Journal of Nuclear Medicine. 2019;60(11):1560-8.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. None.
Author information
Authors and Affiliations
Contributions
K.C. and H.L. drafted the main manuscript text and prepared Fig. 1 and Tables 1, 2, 3, 4, 5. J.K. led the conceptualization and provided valuable supervision and oversight of the project. All authors reviewed the manuscript. Substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data—All authors. Drafting and revising the article—All authors. Final approval of the version to be published—All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lo, H.Z., Choy, K.T. & Kong, J.C.H. FDG-PET/MRI in colorectal cancer care: an updated systematic review. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04460-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04460-z