Abstract
Purpose
To assess the primary safety and oncological outcome of percutaneous cryoablation in patients with non-visceral metastases of the abdominal cavity after prior surgery.
Methods
All patients with non-visceral metastases after prior abdominal surgery, treated with percutaneous cryoablation, and at least one year of follow-up were retrospectively identified. Technical success was achieved if the ice-ball had a minimum margin of 10 mm in three dimensions on the per-procedural CT images. Complications were recorded using the Society of Interventional Radiology (SIR) classification system. Time until disease progression was monitored with follow-up CT and/or MRI. Local control was defined as absence of recurrence at the site of ablation.
Results
Eleven patients underwent cryoablation for 14 non-visceral metastases (mean diameter 20 ± 9 mm). Primary tumor origin was renal cell (n = 4), colorectal (n = 3), granulosa cell (n = 2), endometrium (n = 1) and appendix (n = 1) carcinoma. Treated metastases were localized retroperitoneal (n = 8), intraperitoneal (n = 2), or in the abdominal wall (n = 4). Technical success was achieved in all procedures. After a median follow-up of 27 months (12–38 months), all patients were alive. Local control was observed in 10/14 non-visceral metastases, and the earliest local progression was detected after ten months. No major adverse events occurred. One patient suffered a minor asymptomatic adverse event.
Conclusion
This proof-of-concept study suggests that cryoablation can be a minimal invasive treatment option in a selected group of patients with non-visceral metastases in the abdominal cavity after prior surgery.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abdominal cancers are plagued by a high incidence of recurrences, especially in advanced-stage disease. Tumor recurrence may be due to local recurrence or arise elsewhere in the abdominal cavity. For instance, in patients treated with curative intent for colorectal cancer (CRC), overall recurrence occurs in 17% to 25% [1,2,3]. The recurrence rate in renal cell cancer (RCC) is 21–27% [4,5,6]. Recurrence rates in granulosa cell and endometrial cancers are strongly dependent on stage. Curative treatment of recurrences can be challenging; it aims (1) to improve overall survival, (2) reduce tumor burden, or (3) avoid or delay the start of systemic therapy. Surgery with curative intent is usually performed provided the recurrent disease is limited. However, not all patients are eligible for surgery due to location, proximity to critical structures that can potentially be damaged, extensive prior surgery, morbidity or extent of disease. Besides, patients with an extensive oncological history might benefit from the advantages of minimally invasive therapy, such as the reduced risk of morbidity, shorter hospital admissions, and reduced pain and recovery time.
While heat or cold-based thermal ablation techniques are well-known local treatment options for lesions in the abdominal viscera (e.g., liver/kidney) [7,8,9,10], the clinical benefit of thermal ablation in non-visceral metastases of the abdominal cavity has not yet been established. Cryoablation is a thermal ablation technique wherein tumor tissue is destroyed by extremely cold temperatures. With cryoablation, probes inserted in the tumor cause freezing, resulting in mechanical damage, dehydration and local ischemia, leading to cell death [9, 11]. The formation of an ice-ball creating the ablation zone can be visualized during the freezing process. This real-time visualization limits the risk of damage to adjacent structures and is an important advantage of cryoablation over other thermal ablation techniques.
The aim of this proof-of-concept study is to assess the safety and oncological outcome of percutaneous cryoablation in patients with limited non-visceral metastases of the abdominal cavity after prior surgery.
Materials and methods
Patients
Our Institutional Review Board approved this single-center, retrospective study, and informed consent was waived. From November 2018 to January 2021, all patients treated with cryoablation for non-visceral metastases of the abdominal cavity were included after a follow-up of at least one year. The decision to perform cryoablation was made by a multidisciplinary tumor board (MDT) consisting of a medical oncologist, radiologist, surgical oncologist (urologist, gastrointestinal surgeon, or gynecologist), radiation oncologist, and interventional radiologist. Whole-body CT imaging was performed in all patients to rule out widespread disease. Patients considered eligible for cryoablation by the MDT, had confined non-visceral metastases in the abdominal cavity after prior abdominal surgery, based on pathological proof and/or growth on consecutive imaging. Patients with widespread disease or voluminous non-visceral metastases were not considered eligible. Patient demographics and tumor characteristics, including primary origin, location, and prior local and systemic treatment were recorded.
Cryoablation
All cryoablation procedures were performed, or supervised, by an expert interventional radiologist with 4 to 12 years of experience in thermal ablation. General or epidural anesthesia was administered, and computed tomography (CT) guidance (CT Somatom Sensation Open, Siemens®, Munchen, Germany) was used for realtime evaluation of the procedure. Cryoablation was performed using the Visual ICE™ system (Boston Scientific, USA) with IceForce® or IcePearl® cryoprobes. The number of cryoprobes was determined by the size of the lesion, and inserted with 1 to 2 cm spacing. If deemed necessary, air- or hydrodissection was applied to protect adjacent structures using room air or 5% glucose solution combined with iodine contrast. At least two cycles of freezing, passive thawing, and active thawing were completed with a minimum time of 10 min, 2 min, and 2 min, respectively. Technical success was achieved if the ice-ball had a minimum margin of 10 mm in three dimensions on the periprocedural CT images. Patients stayed overnight for monitoring and were discharged the day after the cryoablation procedure.
Follow-up
Follow-up imaging was scheduled at least at one, six, and twelve months after treatment or when clinical symptoms occurred. The time until disease progression, both local and distant, was monitored using CT and/or magnetic resonance imaging (MRI). Local control was defined as the absence of tumor regrowth within 1 cm of the ablation zone. Distant progression included both new tumor foci at distant sites from the ablation zone and progression of initially stable metastases. If disease progression occurred, further treatment options were discussed in the MDT, and data about the time between ablation and the start of systemic treatment were collected. Adverse events were recorded using the Society of Interventional Radiology (SIR) classification system.
Analysis
Descriptive statistics were used to report on the results. Categorical variables were expressed as frequencies and percentages. Continuous variables were reported as mean and standard deviation or median and range, depending on the distribution of the data.
Results
A total of 11 patients underwent cryoablation for 14 non-visceral metastases. Metastases originated from renal cell (n = 4), colorectal (n = 3), granulosa cell (n = 2), endometrium (n = 1), and appendix (n = 1) carcinoma; patient details are displayed in Table 1. Treated metastases were localized retroperitoneal (n = 8), intraperitoneal (n = 2), or in the abdominal wall (n = 4). Patients were treated for one up to three lesions. The non-visceral metastases had a mean longest diameter of 20 ± 9 mm. 10/11 patients had histopathological evidence available for metastasized disease. Of the treated non-visceral metastases, 6/14 were confirmed by biopsy. Technical success was achieved in all lesions. No major adverse events occurred. One patient developed an asymptomatic pseudocyst adjacent to the ablation zone without the need for further treatment (SIR grade A). Treatment and outcome details are presented in Table 2.
Follow-up
All patients were alive after a median follow-up of 27 months (range 12–38 months), and 10/14 metastases (71%) showed persistent local control. Figures 1 and 2 show examples of successfully treated lesions. The four metastases with local progression were found in three patients (patients 3, 7 and 9 (Table 2)). One patient had local progression and was re-treated with cryoablation 16 months after the initial procedure. Local progression reoccurred ten months after the second cryoablation procedure. The two other patients with local progression had single abdominal wall metastases at the time of the cryoablation. Prior treatment included resection and radiotherapy in one patient, and radiotherapy and radiofrequency ablation in the other patient (patients 9 and 7, respectively). Local control was obtained for 13 and 14 months after cryoablation. Figure 3 displays the images of a patient with local progression.
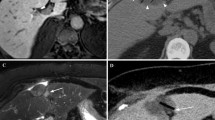
A 57-year-old female patient with a history of mucinous appendix carcinoma treated with a right hemicolectomy, two debulkings including HIPEC, and a liver metastectomy, now presents with a rise in carcinoembryonic antigen (CEA) from 8.0 to 38 µg/L. CT shows a peritoneal metastases of 32 mm located on the diaphragm invading the liver (A). Cryoablation was performed using three needles (B; transverse CT, C; sagittal CT). Coronal CT images show complete ablation after one month (D), and local control after 5 months (E) and 3 years (F) with a stable CEA varying between 5.6 and 7.0 µg/L
A 67-year-old female patient presents with a peritoneal metastases of granulosa cell carcinoma in the epigastric region of 13 mm (A). Cryoablation with one needle with hydrodissection for stomach proximity (B). Follow-up CT shows complete ablation after one month (C) and persistent local control after 27 months (D)
A 76-year-old female patient with a non-visceral metastases of RCC in the abdominal wall of 22 mm (A) after nephrectomy, which was previously treated with SBRT and twice with radiofrequency ablation. Hydrodissection (B) was performed for bowel proximity and one needle was used during cryoablation (C). Follow-up CT shows complete ablation after one month (D), and persistent local control after 8 months (E). Local tumor progression was detected after 14 months (F) which was left untreated and closely monitored with watchful waiting
Five patients developed distant progression outside the ablation zone during follow-up, including one patient who also had local progression. Three out of these five patients had other distant metastases at the time of the cryoablation procedure and the cryoablation was performed for oligoprogression. Three patients started with systemic treatment after distant progression, and the time between the cryoablation procedure and the start of systemic treatment was 2, 14, and 16 months.
Discussion
The present study aims to assess the safety and oncological outcome of this novel strategy in which percutaneous cryoablation was used for patients with limited non-visceral metastases of the abdominal cavity after prior abdominal surgery. This proof-of-concept study demonstrated that percutaneous cryoablation could be a treatment option in carefully selected patients. These patients were treated for a maximum of three non-visceral metastases ranging from 1.1 to 3.9 cm. We observed good local control in 71% of the patients. No major adverse events occurred. One minor adverse event did not require treatment. This suggests cryoablation is a safe procedure for non-visceral abdominal metastases.
Although adequate ablative margins were achieved during the cryoablation procedures, 4 out of 14 metastases showed local progression. An explanation could be that microscopic tumor foci in the vicinity of the metastases were already present at the time of the ablation. Some studies divided local recurrences after cryoablation in procedural and satellite etiology [12, 13]. Bang et al. defined a procedure-related recurrence, as a recurrence in the tumor rim due to inadequate and sublethal temperatures, whereas satellite recurrences were adjacent lesions located within 1 cm of the ablation zone [12]. It suggests that some patients might have local spread of disease around the metastases, which is not visible on imaging and therefore not included in the ablation zone.
In our study, the time to local tumor progression varied between 10 to 14 months. These findings are in contrast with the results of Littrup et al., who have reported an average time to recurrence of 4 months after cryoablation of soft-tissue tumors [13]. We hypothesize, that this could be due to their mean follow-up time of 11 months (and 9 months specifically for retroperitoneal tumors), which could result in the missing of recurrences after 11 months. Other studies found comparable results with ours. Parvinian et al. reported a median time to recurrence of 11 months after cryoablation of lymph node metastases [14]. Similarly, a median progression-free survival of 10 months was found after cryoablation of recurrent CRC in the pelvic cavity [15]. These results suggest that even when local control fails, cryoablation could postpone tumor progression for up to almost a year.
Our findings regarding safety and adverse event rates align with other studies reporting on cryoablation in tumor recurrences in the abdomen. Small series reported 0 to 3% major and 0 to 7% minor adverse event rates [14, 16,17,18]. Whereas Wang et al. found more adverse events with 9% major and 40% minor complications after cryoablation of pelvic CRC recurrences [15]. This difference could be explained by patient selection. Some of their patients were treated to relieve pain; these patients had larger target lesions that were fixed to other structures. In their study, all patients recovered completely, confirming the safety of cryoablation in the abdominal cavity.
This study has several limitations. First, the study had a retrospective design. Second, the patient population was heterogeneous in terms of primary tumor origins, previous treatment, and localizations of treated metastases. Furthermore, histopathological evidence of metastasized disease was missing for 1/11 patients. Being limited to a small study population, this study lacks analysis of risk factors for tumor progression. Nevertheless, we advocate that this minimal invasive treatment should be discussed during MDT meetings in specific patients with small localized metastases of the non-visceral abdomen.
In conclusion, this proof-of-concept study suggests that percutaneous cryoablation can be a minimal invasive treatment option in a selected group of patients with non-visceral metastases of the abdominal cavity after prior surgery. Cryoablation should specifically be considered in patients with metastases that are limited in size and number.
References
T.L. Lash, A.H. Riis, E.B. Ostenfeld, R. Erichsen, M. Vyberg, T.P. Ahern, O. Thorlacius-Ussing, Associations of Statin Use With Colorectal Cancer Recurrence and Mortality in a Danish Cohort, Am J Epidemiol 186(6) (2017) 679-687.
H. Kobayashi, H. Mochizuki, K. Sugihara, T. Morita, K. Kotake, T. Teramoto, S. Kameoka, Y. Saito, K. Takahashi, K. Hase, M. Oya, K. Maeda, T. Hirai, M. Kameyama, K. Shirouzu, T. Muto, Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study, Surgery 141(1) (2007) 67-75.
A. Gunawardene, B. Desmond, A. Shekouh, P. Larsen, E. Dennett, Disease recurrence following surgery for colorectal cancer: five-year follow-up, N Z Med J 131(1469) (2018) 51-58.
S.B. Stewart-Merrill, R.H. Thompson, S.A. Boorjian, S.P. Psutka, C.M. Lohse, J.C. Cheville, B.C. Leibovich, I. Frank, Oncologic Surveillance After Surgical Resection for Renal Cell Carcinoma: A Novel Risk-Based Approach, J Clin Oncol 33(35) (2015) 4151-7.
K. Kobayashi, T. Saito, Y. Kitamura, V. Bilim, T. Toba, T. Kawasaki, N. Hara, T. Tanikawa, Y. Tomita, Clinicopathological features and outcomes in patients with late recurrence of renal cell carcinoma after radical surgery, Int J Urol 23(2) (2016) 132-7.
N.H. Azawi, H. Tesfalem, K.S. Mosholt, P. Høyerup, E.S. Jensen, E. Malchau, M. Fode, Recurrence rates and survival in a Danish cohort with renal cell carcinoma, Dan Med J 63(4) (2016).
C.S. Morris, M.O. Baerlocher, S.R. Dariushnia, E.D. McLoney, N. Abi-Jaoudeh, K. Nelson, M. Cura, A.K. Abdel Aal, J.W. Mitchell, S. Madassery, S. Partovi, T.D. McClure, A.L. Tam, S. Patel, Society of Interventional Radiology Position Statement on the Role of Percutaneous Ablation in Renal Cell Carcinoma: Endorsed by the Canadian Association for Interventional Radiology and the Society of Interventional Oncology, J Vasc Interv Radiol 31(2) (2020) 189–194.e3.
M.R. Callstrom, D.A. Woodrum, F.C. Nichols, J. Palussiere, X. Buy, R.D. Suh, F.G. Abtin, B.B. Pua, D.C. Madoff, S.L. Bagla, D.C. Papadouris, H.C. Fernando, D.E. Dupuy, T.T. Healey, W.H. Moore, T.V. Bilfinger, S.B. Solomon, H. Yarmohammadi, H.J. Krebs, C.J. Fulp, A. Hakime, L. Tselikas, T. de Baere, Multicenter Study of Metastatic Lung Tumors Targeted by Interventional Cryoablation Evaluation (SOLSTICE), J Thorac Oncol 15(7) (2020) 1200-1209.
R.L. Cazzato, J. Garnon, N. Ramamurthy, G. Koch, G. Tsoumakidou, J. Caudrelier, F. Arrigoni, L. Zugaro, A. Barile, C. Masciocchi, A. Gangi, Percutaneous image-guided cryoablation: current applications and results in the oncologic field, Med Oncol 33(12) (2016) 140.
M. Dijkstra, S. Nieuwenhuizen, R.S. Puijk, F.E.F. Timmer, B. Geboers, E.A.C. Schouten, J. Opperman, H.J. Scheffer, J.J.J. Vries, R.J. Swijnenburg, K.S. Versteeg, B.I. Lissenberg-Witte, M.P. van den Tol, M.R. Meijerink, Thermal Ablation Compared to Partial Hepatectomy for Recurrent Colorectal Liver Metastases: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study, Cancers 13(11) (2021).
A.H. Mahnken, A.M. König, J.H. Figiel, Current Technique and Application of Percutaneous Cryotherapy, Rofo 190(9) (2018) 836-846.
H.J. Bang, P.J. Littrup, B.P. Currier, D.J. Goodrich, M. Choi, L.K. Heilbrun, A.C. Goodman, Percutaneous Cryoablation of Metastatic Lesions from Colorectal Cancer: Efficacy and Feasibility with Survival and Cost-Effectiveness Observations, ISRN Minim Invasive Surg 2012 (2012).
P.J. Littrup, H.J. Bang, B.P. Currier, D.J. Goodrich, H.D. Aoun, L.K. Heilbrun, B.A. Adam, Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes, J Vasc Interv Radiol 24(12) (2013) 1817-25.
A. Parvinian, J.J. Schmitz, B.T. Welch, T.D. Atwell, J.M. Morris, D.A. Woodrum, A.N. Kurup, A Single-Institution Experience in Percutaneous Image-Guided Cryoablation of Lymph Node Metastases, AJR Am J Roentgenol 217(1) (2021) 152-156.
Y. Wang, X.H. He, L.C. Xu, H.Z. Huang, G.D. Li, Y.H. Wang, W.T. Li, G.Z. Wang, CT-guided cryoablation for unresectable pelvic recurrent colorectal cancer: a retrospective study, Onco Targets Ther 12 (2019) 1379-1387.
M.E. Nance, M.R. Wakefield, A.P. Bhat, R.M. Davis, Image-guided percutaneous cryo-ablation of peri-urethral unresectable recurrent pelvic malignancy: A case report and brief review, Radiol Case Rep 16(5) (2021) 1227-1232.
G. Tsoumakidou, K. Mandralis, A. Hocquelet, R. Duran, A. Denys, Salvage Lymph-Node Percutaneous Cryoablation: Safety Profile and Oncologic Outcomes, Cardiovasc Intervent Radiol 43(2) (2020) 264-272.
H.J. Bang, P.J. Littrup, D.J. Goodrich, B.P. Currier, H.D. Aoun, L.K. Heilbrun, U. Vaishampayan, B. Adam, A.C. Goodman, Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost-effectiveness for palliation, J Vasc Interv Radiol 23(6) (2012) 770-7.
Funding
No funds, grands, or other support was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant (non-) financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Reijd, D.J., Baetens, T.R., Gomez Munoz, F. et al. Percutaneous cryoablation: a novel treatment option in non-visceral metastases of the abdominal cavity after prior surgery. Abdom Radiol 47, 3345–3352 (2022). https://doi.org/10.1007/s00261-022-03598-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03598-y